Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018-2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice
- PMID: 40002896
- PMCID: PMC11853294
- DOI: 10.3390/biomedicines13020483
Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018-2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice
Abstract
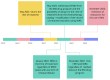
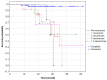
Background: High-grade advanced ovarian, fallopian tube, and primary peritoneal (HGAOC) cancers require both surgical and systemic treatment. The introduction of polyADP-ribose polymerase inhibitors (PARPis) has significantly improved outcomes. This study presents an analysis of HGAOC patients treated at a single center, following updated guidelines. Methods: We observed 437 women newly diagnosed with HGAOC at the Department of Gynecological Oncology between January 2018 and December 2023. Results: Since November 2022, first-line treatment has included bevacizumab and PARPi, regardless of residual disease post-cytoreductive surgery. In both BRCA1/2-mutated and non-mutated groups, PARPi-based regimens increased significantly after May 2021 (p < 0.01). Recurrence number emerged as a strong prognostic factor for survival (p < 0.001), with each recurrence raising mortality risk by 80%. Median survival was 21 months for paclitaxel + platinum derivatives (PC), 27 months for PC + bevacizumab (BEV), and 30 months for PC + BEV + PARPi. Conclusions: The rapid adoption of modern therapies at our center has aligned treatment strategies with HRD status and global standards. However, variations in financial regulations and drug accessibility persist across countries. Despite these challenges, physicians should prioritize the most effective therapies available.
Keywords: HGAOC; OC; high-grade advanced ovarian cancer; molecular diagnostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- The Global Cancer Observatory GLOBOCAN 2020: International Agency Research on Cancer. Int. Agency Res. Cancer. 2020;509:336–343.
-
- McGuire W.P., Hoskins W.J., Brady M.F., Kucera P.R., Partridge E.E., Look K.Y., Clarke-Pearson D.L., Davidson M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. - DOI - PubMed
-
- du Bois A., Lück H.J., Meier W., Adams H.P., Mobus V., Costa S., Bauknecht T., Richter B., Warm M., Schroder W., et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

