Dupilumab in the Treatment of Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and Comorbid Asthma-A Multidisciplinary Monocentric Real-Life Study
- PMID: 40002914
- PMCID: PMC11853246
- DOI: 10.3390/biomedicines13020501
Dupilumab in the Treatment of Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and Comorbid Asthma-A Multidisciplinary Monocentric Real-Life Study
Abstract
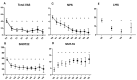
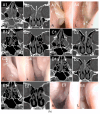
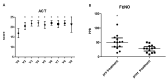
Background: Chronic rhinosinusitis with nasal polyps (CRSwNP) and asthma are mutually correlated with Type-2 inflammation. Dupilumab is effective in uncontrolled and relapsing CRSwNP. However, the precise characterization of Type-2 inflammation and the impact of previous surgery on clinical outcomes need clarification. Methods: We present a prospective observational study on a 38 CRSwNP-patient cohort, whose Type-2 endotype was confirmed after a multidisciplinary approach shared among ENTs, pneumologists and allergologists. Patients were treated with dupilumab and evaluated at 15 days and 1-3-6-12-18-24-30 months, focusing on clinical (VAS, nasal polyp score-NPS), radiological (Lund-Mackay) and quality of life (SNOT-22) parameters, as well olfactory function, asthma control, variation of Type-2 markers and number and extent (ACCESS score) of previous surgeries. Results: We confirmed the efficacy of dupilumab in total and sub-items VAS, NPS, SNOT-22 and sniffing score, as well as Lund-Mackay score improvements, observable and significant after 2 weeks of treatment (p < 0.0001) and long-lasting over 30 months. Good to excellent response criteria to biologic treatment at 6 months was observed in 30/32 patients. Comorbid asthma reached rapid control (p < 0.0001) and exhaled nitric oxide normalization was achieved. One single "not adequate" surgery showed a trend to milder improvement, as well as a higher ACCESS score to better olfactory outcome. Conclusions: The accurate selection of uncontrolled relapsing CRSwNP in terms of Type-2 endotyping by multidisciplinary approach can maximize dupilumab efficacy. The number and extent of previous surgeries may differentiate the response, although this effect is difficult to catch in real life. "Adequate" ESS surgery before dupilumab may drive mostly effective disease control.
Keywords: asthma; biologic treatment; biomarkers; chronic rhinosinusitis; dupilumab; endoscopic sinus surgery; nasal polyps; type-2 inflammation.
Conflict of interest statement
G.L.F. reports a fee as a speaker for GSK, Sanofi, all outside of the submitted work G.G. reports a fee as a speaker for AstraZeneca, Menarini, all outside of the submitted work; F.L.M.R. reports grants, personal fees, and other compensation from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Novartis, and personal fees and grants to support scientific research from Sanofi, all outside of the submitted work. All the other authors declare no conflicts of interest.
Figures



Similar articles
-
Efficacy of dupilumab on chronic rhinosinusitis with nasal polyps and concomitant asthma in biologic-naive and biologic-pretreated patients.Ann Med. 2024 Dec;56(1):2411018. doi: 10.1080/07853890.2024.2411018. Epub 2024 Oct 4. Ann Med. 2024. PMID: 39364704 Free PMC article.
-
Long-term effects of dupilumab on chronic rhinosinusitis with nasal polyps: A step towards clinical remission.World Allergy Organ J. 2025 Jan 16;18(2):101024. doi: 10.1016/j.waojou.2024.101024. eCollection 2025 Feb. World Allergy Organ J. 2025. PMID: 39902112 Free PMC article.
-
Effectiveness of dupilumab versus endoscopic sinus surgery for the treatment of type-2 chronic rhinosinusitis with nasal polyps: a preliminary report.Eur Arch Otorhinolaryngol. 2024 Mar;281(3):1317-1324. doi: 10.1007/s00405-023-08309-x. Epub 2023 Nov 1. Eur Arch Otorhinolaryngol. 2024. PMID: 37910208
-
Biologics for chronic rhinosinusitis.Cochrane Database Syst Rev. 2020 Feb 27;2(2):CD013513. doi: 10.1002/14651858.CD013513.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 12;3:CD013513. doi: 10.1002/14651858.CD013513.pub3. PMID: 32102112 Free PMC article. Updated.
-
Efficacy and safety of dupilumab in the treatment of CRSwNP in the real-life setting: a review of the literature.Eur Arch Otorhinolaryngol. 2024 Oct;281(10):5023-5031. doi: 10.1007/s00405-024-08725-7. Epub 2024 May 19. Eur Arch Otorhinolaryngol. 2024. PMID: 38762844 Review.
References
-
- Bachert C., Han J.K., Desrosiers M., Hellings P.W., Amin N., Leeet S.E., Mullol J., Greos L.S., Bosso J.V., Laidlaw T.M., et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. doi: 10.1016/S0140-6736(19)31881-1. Erratum in Lancet 2019, 394, 1618. - DOI - PubMed
-
- De Corso E., Pasquini E., Trimarchi M., La Mantia I., Pagella F., Ottaviano G., Garzaro M., Pipolo C., Torretta S., Seccia V., et al. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): A multicentric observational Phase IV real-life study (DUPIREAL) Allergy. 2023;78:2669–2683. doi: 10.1111/all.15772. - DOI - PubMed
LinkOut - more resources
Full Text Sources

