Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine
- PMID: 40002954
- PMCID: PMC11852019
- DOI: 10.3390/ani15040472
Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine
Abstract
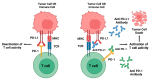
Monoclonal antibody therapy has been a cornerstone of human healthcare for nearly four decades, effectively treating a wide range of diseases including cancers, autoimmune disorders, and inflammatory conditions. However, its application in veterinary medicine is a relatively recent development, offering a promising therapeutic approach for managing chronic diseases in small animals. Dogs and cats, like humans, suffer from chronic conditions such as cancer, arthritis, allergies, and chronic pain, which mAb therapy could potentially address. This review aims to explore the therapeutic potential of mAb therapy in small animal medicine, focusing on currently authorized products, including their mechanisms of action, clinical efficacy, and safety concerns. A comprehensive review of the literature was conducted to evaluate the use of mAbs in veterinary medicine, specifically in the treatment of chronic disorders. While mAb therapy has shown significant benefits in human healthcare, challenges remain in its application to veterinary practice, including safety concerns and the limited availability of approved products. Despite these challenges, mAb therapy holds great promise for improving the management of chronic diseases in animals, with future research and development potentially expanding its clinical use.
Keywords: atopic dermatitis; canine interleukin 31; chronic disorders; immunotherapy; lymphoma; mAb therapy; monoclonal antibody; nerve growth factor; osteoarthritis; pet animals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Research P. Biopharmaceutical Market Size, 2023–2032. 2023. [(accessed on 27 January 2025)]. Available online: https://www.precedenceresearch.com/biopharmaceutical-market.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

