Protocells Either Synchronize or Starve
- PMID: 40003151
- PMCID: PMC11854096
- DOI: 10.3390/e27020154
Protocells Either Synchronize or Starve
Abstract
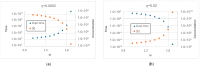
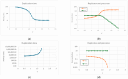
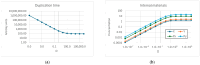
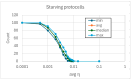
Two different processes take place in self-reproducing protocells, i.e., (i) cell reproduction by fission and (ii) duplication of the genetic material. One major problem is indeed that of assuring that the two processes take place at the same pace, i.e., that they synchronize, which is a necessary condition for sustainable growth. In previous theoretical works, using dynamical models, we had shown that such synchronization can spontaneously emerge, generation after generation, under a broad set of hypotheses about the architecture of the protocell, the nature of the self-replicating molecules, and the types of kinetic equations. However, an important class of cases (quadratic or higher-order self-replication) did not synchronize in the models we had used, but could actually lead to divergence of the concentration of replicators. We show here that this behavior is due to a simplification of the previous models, i.e., the "buffering" hypothesis, which assumes instantaneous equilibrium of the internal and external concentrations of those compounds which can cross the cell membrane. That divergence disappears if we make use of more realistic dynamical models, with finite transmembrane diffusion rates of the precursors of replicators.
Keywords: Fick’s law; chemical kinetics; diffusion rate; self-replication; self-reproduction; transmembrane diffusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. Garland Science; New York, NY, USA: 2014.
-
- Luisi P.L. The Emergence of Life: From Chemical Origins to Synthetic Biology. Cambridge University Press; New York, NY, USA: 2007.
-
- Rasmussen S., Bedau M.A., Chen L., Deamer D., Krakauer D.C., Packard N.H., Stadler P.F. Protocells. MIT Press; Cambridge, MA, USA: 2008.
-
- Israelachvili J.N., Mitchell D.J., Ninham B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2. 1976;72:1525. doi: 10.1039/f29767201525. - DOI
LinkOut - more resources
Full Text Sources

