Utility Assessment of Isolated Starch and Extract from Thai Yam (Dioscorea hispida Dennst.) for Cosmetic via In Vitro and In Vivo Studies
- PMID: 40003560
- PMCID: PMC11856013
- DOI: 10.3390/life15020151
Utility Assessment of Isolated Starch and Extract from Thai Yam (Dioscorea hispida Dennst.) for Cosmetic via In Vitro and In Vivo Studies
Abstract
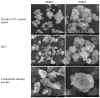
In Thailand, wild yam, or Dioscorea hispida Dennst., is a starchy crop that is usually underutilized in industry. The purpose of this study was to isolate the starch and extract the phytochemical from D. hispida and use them in cosmetics. Starch was used instead of talcum, which can cause pulmonary talcosis in dusting powder formulas (DP 1-5). GC-MS was used to identify the bioactive components present in the ethanolic extract of D. hispida. The main compounds were identified as 9,12-octadecadienoic acid (Z,Z)- (6.51%), stigmasta-5,22-dien-3-ol, (3.beta.,22E)- (6.41%), linoleic acid ethyl ester (5.72%), (Z,Z)-9,12-octadeca-dienoic acid, 2,3-dihydroxy-propyl (3.89%), and campesterol (3.40%). Then, the extract was used as an ingredient in facial sleeping mask gel formulas (SM 1-SM 5). Stability tests, physical characteristics, enzyme inhibitions, and sensitization dermal toxicity tests were used to evaluate the DP and SM formulations. The results showed that the fresh tubers of D. hispida showed a 12.5% w/w starch content. The findings demonstrated that starch powder had a restricted size distribution, ranging from 2 to 4 μm, and a smooth surface that was polygonal. Following stability testing, the color, odor, size, and flowability of all DP formulations did not significantly differ. The SEM investigation revealed that DP particles were homogenous. For the sensitization dermal toxicity test, DP denoted no erythema or skin irritation in the guinea pigs. After stability testing, the colors of the SM formulas were deeper, and their viscosity slightly increased. The pH did not significantly change. After the stability test, SM formulas that contained Glycyrrhiza glabra and D. hispida extracts exhibited stable tyrosinase and elastase inhibitory activities, respectively. In the sensitization dermal toxicity test, guinea pigs showed skin irritation at level 2 (not severe) from SM, indicating that redness developed. All of these findings indicate that D. hispida is a plant that has potential for use in the cosmetics industry. Furthermore, D. hispida starch can be made into a beauty dusting powder, and more research should be conducted to develop an effective remedy for patients or those with skin problems.
Keywords: Dioscorea hispida; cosmetic; dusting powder; elastase; facial sleeping mask; tyrosinase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Final report of the amended safety assessment of Dioscorea Villosa (Wild Yam) root extract.Int J Toxicol. 2004;23 Suppl 2:49-54. doi: 10.1080/10915810490499055. Int J Toxicol. 2004. PMID: 15513824 Review.
-
Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers.Polymers (Basel). 2021 Feb 15;13(4):584. doi: 10.3390/polym13040584. Polymers (Basel). 2021. PMID: 33672030 Free PMC article.
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
-
Amended final report on the safety assessment of Oryza Sativa (rice) Bran Oil, Oryza Sativa (rice) Germ Oil, Rice Bran Acid,Oryza Sativa (rice) Bran Wax, Hydrogenated Rice Bran Wax, Oryza Sativa (rice)Bran Extract, Oryza Sativa (rice) Extract, Oryza Sativa (rice) Germ Powder, Oryza Sativa (rice) Starch, Oryza Sativa (rice) Bran, Hydrolyzed Rice Bran Extract, Hydrolyzed Rice Bran Protein, Hydrolyzed Rice Extract, and Hydrolyzed Rice Protein.Int J Toxicol. 2006;25 Suppl 2:91-120. doi: 10.1080/10915810600964626. Int J Toxicol. 2006. PMID: 17090480 Review.
-
Gene Expression Profiling associated with Hepatoxicity in Pregnant Rats treated with Ubi Gadong (Dioscorea hispida) Extract.Int J Biomed Sci. 2017 Mar;13(1):26-34. Int J Biomed Sci. 2017. PMID: 28533734 Free PMC article.
References
-
- Korkina L., Kostyuk V., Potapovich A., Mayer W., Talib N., De Luca C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics. 2018;5:32. doi: 10.3390/cosmetics5020032. - DOI
-
- Chen D., Mubeen B., Hasnain A., Rizwan M., Adrees M., Naqvi S.A.H., Iqbal S., Kamran M., El-Sabrout A.M., Elansary H.O., et al. Role of Promising Secondary Metabolites to Confer Resistance Against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant Sci. 2022;13:881032. doi: 10.3389/fpls.2022.881032. - DOI - PMC - PubMed
-
- Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392.
-
- Gyawali R., Paudel P.N. Herbal remedies in Cosmeceuticals formulation: A review on Nepalese perspectives. Annapurna J. Health Sci. 2022;2:59–65. doi: 10.52910/ajhs.66. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

