Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack
- PMID: 40003847
- PMCID: PMC11856983
- DOI: 10.3390/insects16020218
Fall Armyworm-Induced Secondary Metabolites in Sorghum Defend Against Its Attack
Abstract
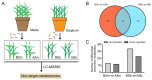
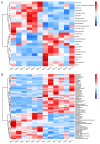
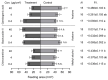
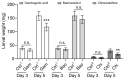
The fall armyworm (FAW), Spodoptera frugiperda, is one of the major agricultural pests that has invaded China. The FAW is a polyphagous insect with the gramineous crop sorghum being a key host plant. However, the basis of sorghum's chemical defense against FAW feeding is still unclear. In this study, we investigated the potential defensive mechanism of sorghum against this insect species. It was found that FAW larvae preferred maize over sorghum, the selection and damage rates for sorghum plants by larvae were significantly lower than those of maize plants, and feeding on sorghum restricted larval weight. The non-target metabolomics revealed that the feeding of FAW larvae altered the plant secondary metabolite spectra in maize and sorghum, resulting in species-specific differential secondary metabolites (DSMs). Of these, 19 DSMs were specific in maize, and 51 in sorghum, and only 6 were found in both species. Two-choice and no-choice feeding assays found that gambogenic acid and chimonanthine, two DSMs unique to sorghum, were found to deter larval feeding and decrease the larval weight. These findings reveal that the defense of sorghum against FAW is regulated by changing the response spectra of secondary metabolites and that the induced metabolites have a defensive function by acting as antifeedants, which provides new insights into employing bioactive plant compounds against polyphagous insects.
Keywords: Spodoptera frugiperda; antifeedant; defense mechanism; secondary metabolites; sorghum.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Fall Armyworm Frass Induce Sorghum Defenses Against Insect Herbivores.J Chem Ecol. 2025 Mar 13;51(2):39. doi: 10.1007/s10886-025-01591-5. J Chem Ecol. 2025. PMID: 40080257
-
Beyond Bites: Differential Role of Fall Armyworm Oral Secretions and Saliva in Modulating Sorghum Defenses.Mol Plant Microbe Interact. 2024 Mar;37(3):232-238. doi: 10.1094/MPMI-12-23-0213-FI. Epub 2024 Mar 28. Mol Plant Microbe Interact. 2024. PMID: 38240672
-
Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm.Front Plant Sci. 2022 Nov 25;13:1019266. doi: 10.3389/fpls.2022.1019266. eCollection 2022. Front Plant Sci. 2022. PMID: 36507437 Free PMC article.
-
Exploring Metabolomics to Innovate Management Approaches for Fall Armyworm (Spodoptera frugiperda [J.E. Smith]) Infestation in Maize (Zea mays L.).Plants (Basel). 2024 Sep 2;13(17):2451. doi: 10.3390/plants13172451. Plants (Basel). 2024. PMID: 39273935 Free PMC article. Review.
-
Opportunities and Scope for Botanical Extracts and Products for the Management of Fall Armyworm (Spodoptera frugiperda) for Smallholders in Africa.Plants (Basel). 2020 Feb 6;9(2):207. doi: 10.3390/plants9020207. Plants (Basel). 2020. PMID: 32041322 Free PMC article. Review.
Cited by
-
Fall Armyworm Frass Induce Sorghum Defenses Against Insect Herbivores.J Chem Ecol. 2025 Mar 13;51(2):39. doi: 10.1007/s10886-025-01591-5. J Chem Ecol. 2025. PMID: 40080257
References
-
- Taiz L., Zeiger E., Møller I.M., Murphy A. Plant Physiology and Development. 6th ed. Sinauer Associates; Sunderland, MA, USA: 2015. pp. 1–761.
Grants and funding
- 202304051001006/the Science and Technology Innovation Teams of Shanxi Province
- 32202391/the National Natural Science Foundation of China
- 2023BQ12/the PhD Research Launch Project of Shanxi Agricultural University
- 202203021212419/the Fundamental Research Program of Shanxi Province
- GLS-gp-202402/the National Fund Cultivation Project of Sorghum Research Institute, Shanxi Agricultural University
LinkOut - more resources
Full Text Sources

