The Role of the Fox Gene in Breast Cancer Progression
- PMID: 40003882
- PMCID: PMC11855465
- DOI: 10.3390/ijms26041415
The Role of the Fox Gene in Breast Cancer Progression
Abstract
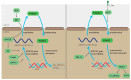
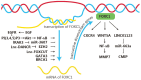
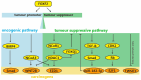
Forkhead box (FOX) genes are a family of transcription factors that participate in many biological activities, from early embryogenesis to the formation of organs, and from regulation of glucose metabolism to regulation of longevity. Given the extensive influence in the multicellular process, FOX family proteins are responsible for the progression of many types of cancers, especially lung cancer, breast cancer, prostate cancer, and other cancers. Breast cancer is the most common cancer among women, and 2.3 million women were diagnosed in 2020. So, various drugs targeting the FOX signaling pathway have been developed to inhibit breast cancer progression. While the role of the FOX family gene in cancer development has not received enough attention, discovering more potential drugs targeting the FOX signaling pathway is urgently demanded. Here, we review the main members in the FOX gene family and summarize their signaling pathway, including the regulation of the FOX genes and their effects on breast cancer progression. We hope this review will emphasize the understanding of the role of the FOX gene in breast cancer and inspire the discovery of effective anti-breast cancer medicines targeting the FOX gene in the future.
Keywords: breast cancer; drug resistance; forkhead box (FOX).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Unravelling the therapeutic potential of forkhead box proteins in breast cancer: An update (Review).Oncol Rep. 2024 Jul;52(1):92. doi: 10.3892/or.2024.8751. Epub 2024 Jun 7. Oncol Rep. 2024. PMID: 38847267 Free PMC article. Review.
-
The roles of FOX proteins in virus-associated cancers.J Cell Physiol. 2019 Apr;234(4):3347-3361. doi: 10.1002/jcp.27295. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30362516 Review.
-
The dual role of FOXF2 in regulation of DNA replication and the epithelial-mesenchymal transition in breast cancer progression.Cell Signal. 2016 Oct;28(10):1502-19. doi: 10.1016/j.cellsig.2016.06.021. Epub 2016 Jul 1. Cell Signal. 2016. PMID: 27377963 Free PMC article.
-
Overexpression of Forkhead Box L1 (FOXL1) Inhibits the Proliferation and Invasion of Breast Cancer Cells.Oncol Res. 2017 Jul 5;25(6):959-965. doi: 10.3727/096504016X14803482769179. Epub 2016 Nov 29. Oncol Res. 2017. PMID: 27938507 Free PMC article.
-
An Integrated Study on the Differential Expression of the FOX Gene Family in Cancer and Their Response to Chemotherapy Drugs.Genes (Basel). 2022 Sep 28;13(10):1754. doi: 10.3390/genes13101754. Genes (Basel). 2022. PMID: 36292640 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

