Combining Sulfonylureas with Anticancer Drugs: Evidence of Synergistic Efficacy with Doxorubicin In Vitro and In Vivo
- PMID: 40003896
- PMCID: PMC11855866
- DOI: 10.3390/ijms26041429
Combining Sulfonylureas with Anticancer Drugs: Evidence of Synergistic Efficacy with Doxorubicin In Vitro and In Vivo
Abstract
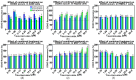
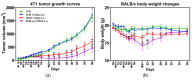
Sulfonylureas (SUs)-a class of drugs primarily used to treat type 2 diabetes-have recently attracted interest for their potential anticancer properties. While some studies have explored the chemical modification or design of new SU derivatives, our work instead centers on biological evaluations of all commercially available SUs in combination with doxorubicin (DOXO). These antidiabetic agents act by stimulating insulin secretion via KATP channel inhibition, and because KATP channels share structural features with ATP-binding cassette (ABC) transporters involved in multidrug resistance (e.g., P-glycoprotein, MRP1, and MRP2), SUs may also reduce cancer cell drug efflux. In this study, we systematically examined each commercially available SU for potential synergy with DOXO in a panel of human cancer cell lines. Notably, combining DOXO with glimepiride (GLIM), the newest SU, results in a 4.4-fold increase in cytotoxicity against MCF-7 breast cancer cells relative to DOXO alone. Mechanistic studies suggest that the observed synergy may arise from increased intracellular accumulation of DOXO. Preliminary in vivo experiments support these findings, showing that DOXO (5 mg/kg, i.v.) plus GLIM (4 mg/kg, i.p.) is more effective at inhibiting 4T1 tumor growth in mice than DOXO alone. Additionally, we show that adding a small amount of the surfactant Tween-80 to culture media affects SU binding to bovine serum albumin (BSA), potentially unmasking anticancer effects of SUs that strongly bind to proteins. Overall, these results underscore the potential of repurposing existing SUs to enhance standard chemotherapy regimens.
Keywords: combination index; combination therapy; doxorubicin; drug repurposing; sulfonylureas; synergistic cytotoxicity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
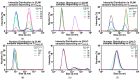

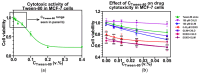
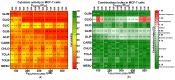
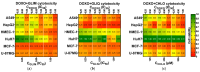




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

