Engineering of Genetically Encoded Bright Near-Infrared Fluorescent Voltage Indicator
- PMID: 40003908
- PMCID: PMC11855178
- DOI: 10.3390/ijms26041442
Engineering of Genetically Encoded Bright Near-Infrared Fluorescent Voltage Indicator
Abstract
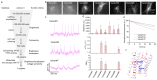
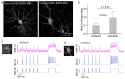
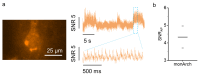
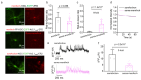
Genetically encoded voltage indicators (GEVIs) allow for the cell-type-specific real-time imaging of neuronal membrane potential dynamics, which is essential to understanding neuronal information processing at both cellular and circuit levels. Among GEVIs, near-infrared-shifted GEVIs offer faster kinetics, better tissue penetration, and compatibility with optogenetic tools, enabling all-optical electrophysiology in complex biological contexts. In our previous work, we employed the directed molecular evolution of microbial rhodopsin Archaerhodopsin-3 (Arch-3) in mammalian cells to develop a voltage sensor called Archon1. Archon1 demonstrated excellent membrane localization, signal-to-noise ratio (SNR), sensitivity, kinetics, and photostability, and full compatibility with optogenetic tools. However, Archon1 suffers from low brightness and requires high illumination intensities, which leads to tissue heating and phototoxicity during prolonged imaging. In this study, we aim to improve the brightness of this voltage sensor. We performed random mutation on a bright Archon derivative and identified a novel variant, monArch, which exhibits satisfactory voltage sensitivity (4~5% ΔF/FAP) and a 9-fold increase in basal brightness compared with Archon1. However, it is hindered by suboptimal membrane localization and compromised voltage sensitivity. These challenges underscore the need for continued optimization to achieve an optimal balance of brightness, stability, and functionality in rhodopsin-based voltage sensors.
Keywords: brightness; directed molecular evolution; genetically encoded voltage indicator; near infrared; phototoxicity; rhodopsin.
Conflict of interest statement
B.S. is a founder of CellSorter start-up company and was employed by CellSorter Scientific company. The remaining authors declare that the research study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Characterization of two near-infrared genetically encoded voltage indicators.Neurophotonics. 2024 Apr;11(2):024201. doi: 10.1117/1.NPh.11.2.024201. Epub 2023 Dec 11. Neurophotonics. 2024. PMID: 38090225 Free PMC article.
-
Temporal dynamics of microbial rhodopsin fluorescence reports absolute membrane voltage.Biophys J. 2014 Feb 4;106(3):639-48. doi: 10.1016/j.bpj.2013.11.4493. Biophys J. 2014. PMID: 24507604 Free PMC article.
-
Enhanced Archaerhodopsin Fluorescent Protein Voltage Indicators.PLoS One. 2013 Jun 19;8(6):e66959. doi: 10.1371/journal.pone.0066959. Print 2013. PLoS One. 2013. PMID: 23840563 Free PMC article.
-
Voltage imaging with genetically encoded indicators.Curr Opin Chem Biol. 2017 Aug;39:1-10. doi: 10.1016/j.cbpa.2017.04.005. Epub 2017 Apr 28. Curr Opin Chem Biol. 2017. PMID: 28460291 Free PMC article. Review.
-
Genetically Encoded Voltage Indicators.Adv Exp Med Biol. 2021;1293:209-224. doi: 10.1007/978-981-15-8763-4_12. Adv Exp Med Biol. 2021. PMID: 33398815 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

