The Crosstalk Between Endothelial Cells, Smooth Muscle Cells, and Macrophages in Atherosclerosis
- PMID: 40003923
- PMCID: PMC11855868
- DOI: 10.3390/ijms26041457
The Crosstalk Between Endothelial Cells, Smooth Muscle Cells, and Macrophages in Atherosclerosis
Abstract
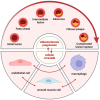
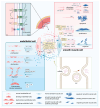
Atherosclerosis (AS) is a chronic inflammatory vascular disease closely tied to cellular metabolism. Recent genome-wide association study data have suggested the significant roles of endothelial cells, smooth muscle cells, and macrophages in the regression and exacerbation of AS. However, the impact of cellular crosstalk and cellular metabolic derangements on disease progression in AS is vaguely understood. In this review, we analyze the roles of the three cell types in AS. We also summarize the crosstalk between the two of them, and the associated molecules and consequences involved. In addition, we emphasize potential therapeutic targets and highlight the importance of the three-cell co-culture model and extracellular vesicles in AS-related research, providing ideas for future studies.
Keywords: atherosclerosis; endothelial cells; macrophages; smooth muscle cells.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

