Evaluation of Small-Molecule Candidates as Modulators of M-Type K+ Currents: Impacts on Current Amplitude, Gating, and Voltage-Dependent Hysteresis
- PMID: 40003973
- PMCID: PMC11855363
- DOI: 10.3390/ijms26041504
Evaluation of Small-Molecule Candidates as Modulators of M-Type K+ Currents: Impacts on Current Amplitude, Gating, and Voltage-Dependent Hysteresis
Abstract
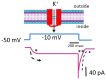
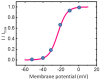
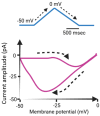
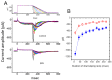
The core subunits of the KV7.2, KV7.3, and KV7.5 channels, encoded by the KCNQ2, KCNQ3, and KCNQ5 genes, are expressed across various cell types and play a key role in generating the M-type K+ current (IK(M)). This current is characterized by an activation threshold at low voltages and displays slow activation and deactivation kinetics. Variations in the amplitude and gating kinetics of IK(M) can significantly influence membrane excitability. Notably, IK(M) demonstrates distinct voltage-dependent hysteresis when subjected to prolonged isosceles-triangular ramp pulses. In this review, we explore various small-molecule modulators that can either inhibit or enhance the amplitude of IK(M), along with their perturbations on its gating kinetics and voltage-dependent hysteresis. The inhibitors of IK(M) highlighted here include bisoprolol, brivaracetam, cannabidiol, nalbuphine, phenobarbital, and remdesivir. Conversely, compounds such as flupirtine, kynurenic acid, naringenin, QO-58, and solifenacin have been shown to enhance IK(M). These modulators show potential as pharmacological or therapeutic strategies for treating certain disorders linked to gain-of-function or loss-of-function mutations in M-type K+ (KV7x or KCNQx) channels.
Keywords: M-type (KV7x or KCNQx) channel; M-type K+ current; current kinetics; small-molecule modulator.
Conflict of interest statement
The authors declare no conflicts of interests that are directly relevant to this work. The content and writing of this paper are solely the responsibility of the authors.
Figures
Similar articles
-
Evidence for Dual Activation of IK(M) and IK(Ca) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol[1,5-a]pyrimidin-7-one).Int J Mol Sci. 2022 Jun 24;23(13):7042. doi: 10.3390/ijms23137042. Int J Mol Sci. 2022. PMID: 35806047 Free PMC article.
-
The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action.Int J Mol Sci. 2021 Nov 17;22(22):12399. doi: 10.3390/ijms222212399. Int J Mol Sci. 2021. PMID: 34830281 Free PMC article.
-
Evidence for Inhibitory Perturbations on the Amplitude, Gating, and Hysteresis of A-Type Potassium Current, Produced by Lacosamide, a Functionalized Amino Acid with Anticonvulsant Properties.Int J Mol Sci. 2022 Jan 21;23(3):1171. doi: 10.3390/ijms23031171. Int J Mol Sci. 2022. PMID: 35163091 Free PMC article.
-
Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents.Int J Mol Sci. 2022 Aug 21;23(16):9453. doi: 10.3390/ijms23169453. Int J Mol Sci. 2022. PMID: 36012718 Free PMC article. Review.
-
The therapeutic potential of neuronal KCNQ channel modulators.Expert Opin Ther Targets. 2003 Dec;7(6):737-48. doi: 10.1517/14728222.7.6.737. Expert Opin Ther Targets. 2003. PMID: 14640909 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

