Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy
- PMID: 40004014
- PMCID: PMC11855499
- DOI: 10.3390/ijms26041548
Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy
Abstract
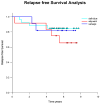
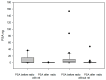
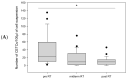
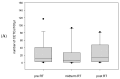
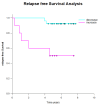
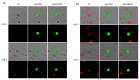
Prostate cancer is a leading cause of cancer-related mortality in men, with radiotherapy (RT) playing a pivotal role in treatment. However, reliable biomarkers for assessing relapse risk following RT remain scarce. This study aimed to evaluate circulating epithelial tumor cells (CETC/CTC) as potential biomarkers for assessing relapse risk in prostate cancer patients undergoing RT. Peripheral blood samples were collected from 52 prostate cancer patients, and CETC/CTC were detected using the EpCAM surface marker. Patients received definitive, adjuvant, or salvage RT, and CETC/CTC counts were measured before, at mid-treatment, and at the end of RT. The association between changes in CETC/CTC counts and relapse risk was examined. CETC/CTC were detected in 96% of patients prior to RT. A significant reduction in CETC/CTC counts during RT, particularly in patients who had undergone surgery, was associated with a lower relapse risk. In contrast, an increase in CETC/CTC counts during or after RT was associated with a higher relapse risk (hazard ratio = 8.8; p = 0.002). Furthermore, 36% of patients receiving adjuvant RT and 14% of those receiving definitive RT relapsed, with a higher risk observed in patients showing increasing CETC/CTC counts during RT. Among patients receiving salvage RT, 18% relapsed, though changes in CETC/CTC counts were less significantly associated with relapse. Monitoring CETC/CTC levels during RT offers important prognostic insights into relapse risk in prostate cancer patients. While changes in CETC/CTC counts correlated with relapse, PSA levels measured during the study did not reliably reflect relapse risk in this cohort. CETC/CTC shows promise as a prognostic marker, though further studies are required to validate its clinical superiority over PSA.
Keywords: PSA; circulating epithelial tumor cells; prognostic biomarker; prostate cancer; radiotherapy; risk stratification.
Conflict of interest statement
KP is the holder of the patents for the herein described method maintrac® to detect CETC/CTC. The other authors declare no conflicts of interest.
Figures






References
-
- Leslie S.W., Soon-Sutton T.L., Skelton W.P. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2022. Prostate Cancer. StatPearls Publishing Copyright © 2022.
-
- Zelefsky M.J., Pei X., Chou J.F., Schechter M., Kollmeier M., Cox B., Yamada Y., Fidaleo A., Sperling D., Happersett L., et al. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur. Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. - DOI - PMC - PubMed
-
- Bolla M., van Poppel H., Collette L., van Cangh P., Vekemans K., Da Pozzo L., de Reijke T.M., Verbaeys A., Bosset J.-F., van Velthoven R., et al. Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

