The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation
- PMID: 40004018
- PMCID: PMC11855581
- DOI: 10.3390/ijms26041552
The Simultaneous Deletion of pH-Sensing Receptors GPR4 and OGR1 (GPR68) Ameliorates Colitis with Additive Effects on Multiple Parameters of Inflammation
Abstract
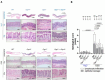
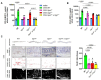
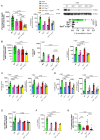
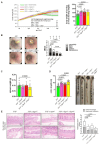
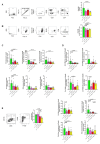
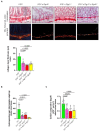
G protein-coupled receptors (GPRs), including pro-inflammatory GPR4 and ovarian cancer GPR1 (OGR1/GPR68), are involved in the pH sensing of the extracellular space and have been implicated in inflammatory bowel disease (IBD). Previous data show that a loss of GPR4 or OGR1 independently is associated with reduced intestinal inflammation in mouse models of experimental colitis. In the present manuscript, we investigated the impact of the simultaneous loss of GPR4 and OGR1 in animal models of IBD. To study the effects of combined loss of Gpr4 Ogr1 in IBD we used the well-established acute dextran sodium sulfate (DSS) and spontaneous Il10-/- murine colitis models. Disease severity was assessed using multiple clinical scores (e.g., body weight loss, disease activity score, murine endoscopic index of colitis severity (MEICS) and histological analyses). Real-time quantitative polymerase chain reaction (qPCR), Western blot, and flow cytometry were used to investigate changes in pro-inflammatory cytokines expression and immune cells infiltration. We found that a combined loss of GPR4 and OGR1 significantly reduces colon inflammation in IBD relative to single deficiencies as evidenced by reduced body weight loss, disease score, CD4/CD8 ratio, and Il1β, Il6, and Tnf in the colon. Similarly, in the II10 deficiency model, the inflammation was significantly ameliorated upon the simultaneous deletion of GPR4 and OGR1, evidenced by a reduction in the MEICS score, colon length, Tnf and Il1β measurements, and a decrease in the number of macrophages in the colon, as compared to single deletions. Importantly, hydroxyproline levels were decreased close to baseline in Il10-/- × Gpr4-/- × Ogr1-/- mice. Our findings demonstrate that the simultaneous loss of GRP4 and OGR1 functions exerts an additive effect on multiple parameters associated with colonic inflammation. These results further reinforce the hypothesis that chronic inflammatory acidosis is a driver of fibrosis and is dependent on GPR4 and OGR1 signaling. The inhibition of both GPR4 and OGR1 by pH-sensing receptor modulators may constitute as a potential therapeutic option for IBD, as both pH-sensing receptors appear to sustain inflammation by acting on complementary pro-inflammatory pathways.
Keywords: GPR4; OGR1; inflammatory bowel disease; pH-sensing G protein-coupled receptors.
Conflict of interest statement
The authors declare no competing interests. G.R. discloses grant support from AbbVie, Ardeypharm, MSD, FALK, Flamentera, Novartis, Roche, Tillots, UCB, and Zeller. All other authors have nothing to disclose.
Figures



References
-
- Wyder L., Suply T., Ricoux B., Billy E., Schnell C., Baumgarten B.U., Maira S.M., Koelbing C., Ferretti M., Kinzel B., et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis. 2011;14:533–544. doi: 10.1007/s10456-011-9238-9. - DOI - PubMed
-
- Miltz W., Velcicky J., Dawson J., Littlewood-Evans A., Ludwig M.G., Seuwen K., Feifel R., Oberhauser B., Meyer A., Gabriel D., et al. Design and synthesis of potent and orally active GPR4 antagonists with modulatory effects on nociception, inflammation, and angiogenesis. Bioorg. Med. Chem. 2017;25:4512–4525. doi: 10.1016/j.bmc.2017.06.050. - DOI - PubMed
-
- Jasso G.J., Jaiswal A., Varma M., Laszewski T., Grauel A., Omar A., Silva N., Dranoff G., Porter J.A., Mansfield K., et al. Colon stroma mediates an inflammation-driven fibroblastic response controlling matrix remodeling and healing. PLoS Biol. 2022;20:e3001532. doi: 10.1371/journal.pbio.3001532. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

