Vault Particles in Cancer Progression, Multidrug Resistance, and Drug Delivery: Current Insights and Future Applications
- PMID: 40004027
- PMCID: PMC11855390
- DOI: 10.3390/ijms26041562
Vault Particles in Cancer Progression, Multidrug Resistance, and Drug Delivery: Current Insights and Future Applications
Abstract
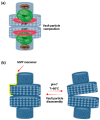
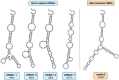
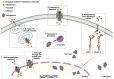
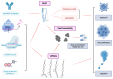
Vault particles (VPs) are highly conserved large ribonucleoprotein complexes found exclusively in eukaryotes. They play critical roles in various cellular processes, but their involvement in cancer progression and multidrug resistance (MDR) is the most extensively studied. VPs are composed of the major vault protein (MVP), vault RNAs (vtRNAs), vault poly (ADP-ribose) polymerase, and telomerase-associated protein-1. These components are involved in the regulation of signaling pathways that affect tumor survival, proliferation, and metastasis. MVP has been associated with aggressive tumor phenotypes, while vtRNAs modulate cell proliferation, apoptosis, and autophagy. VPs also contribute to MDR by sequestering chemotherapeutic agents, altering their accumulation in the nucleus, and regulating lysosomal dynamics. Furthermore, small vault RNA-derived fragments participate in gene silencing and intercellular communication, reinforcing the role of precursors of vtRNAs in cancer development. Beyond their biological roles, VPs present a promising platform for drug delivery, due to their unique ability to encapsulate a wide range of biomolecules and therapeutic agents, followed by controlled release. This review compiles data from PubMed and Scopus, with a literature search conducted up until December 2024, highlighting current knowledge regarding VPs and their crucial involvement in cancer-related mechanisms and their applications in overcoming cancer drug resistance.
Keywords: cancer progression; drug delivery nanoparticle; major vault protein (MVP); multidrug resistance (MDR); small vault RNA (svtRNA); vault RNA (vtRNAs); vault particle (VP).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

