Isoliquiritigenin Ameliorates High-Fat Diet-Induced Obesity in Mice by Activating Brown Adipose Tissue
- PMID: 40004080
- PMCID: PMC11855605
- DOI: 10.3390/ijms26041616
Isoliquiritigenin Ameliorates High-Fat Diet-Induced Obesity in Mice by Activating Brown Adipose Tissue
Abstract
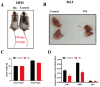
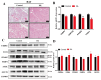
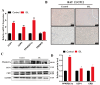
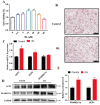
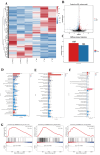
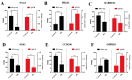
Brown adipose tissue (BAT) is a critical regulator of non-shivering thermogenesis and energy expenditure, offering significant potential for addressing obesity and associated metabolic disorders. Isoliquiritigenin (ISL), a natural flavonoid, has shown promising therapeutic effects in lipid metabolism-related diseases. This study aimed to explore the effects of ISL on lipid metabolism and obesity using a high-fat-diet (HFD)-induced obesity model in mice. Mice were subjected to an HFD and treated with ISL via gavage. The results demonstrated that ISL treatment significantly reduced HFD-induced weight gain and upregulated the expression of key thermogenic genes, suggesting enhanced BAT activity and thermogenesis. In vitro experiments using C3H10-T1/2 cells further supported these findings, as ISL treatment markedly increased the expression of UCP1 and PPARGC1a, which are critical regulators of thermogenesis. To elucidate the molecular mechanisms underlying ISL's effects, we conducted a transcriptomic analysis of BAT from ISL-treated mice. Pathway enrichment analysis revealed that differentially expressed genes were predominantly associated with metabolic processes, including the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, and fatty acid degradation. These pathways are integral to energy metabolism and thermogenesis, providing mechanistic insights into ISL's anti-obesity effects. Additionally, ISL treatment significantly downregulated the expression of NNAT and SGK1, genes implicated in lipid metabolism and energy homeostasis. These findings suggest that ISL modulates BAT function by regulating the expression of these genes, thereby influencing lipid deposition and thermogenic capacity. In summary, this study suggests that ISL treatment has the potential to mitigate HFD-induced obesity by promoting BAT thermogenesis and modulating lipid metabolism. The molecular mechanisms involve the regulation of key metabolic pathways and genes, such as NNAT and SGK1, highlighting ISL's potential as a therapeutic agent for obesity and related metabolic disorders.
Keywords: brown adipose tissue; high-fat diet; isoliquiritigenin; obesity; thermogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Chinese medicine Jinlida granules improve high-fat-diet induced metabolic disorders via activation of brown adipose tissue in mice.Biomed Pharmacother. 2019 Jun;114:108781. doi: 10.1016/j.biopha.2019.108781. Epub 2019 Mar 20. Biomed Pharmacother. 2019. PMID: 30903919
-
Differential effects of milk, yogurt, and cheese on energy homeostasis and brown adipose tissue phenotype in high-fat diet-induced obese mice.Food Funct. 2024 Sep 30;15(19):9833-9848. doi: 10.1039/d4fo02201g. Food Funct. 2024. PMID: 39230108
-
Exendin-4 improves thermogenic capacity by regulating fat metabolism on brown adipose tissue in mice with diet-induced obesity.Ann Clin Lab Sci. 2015 Spring;45(2):158-65. Ann Clin Lab Sci. 2015. PMID: 25887869
-
Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity.Biochimie. 2017 Mar;134:62-70. doi: 10.1016/j.biochi.2016.09.007. Epub 2016 Sep 10. Biochimie. 2017. PMID: 27621146 Review.
-
UCP1-independent thermogenesis.Biochem J. 2020 Feb 14;477(3):709-725. doi: 10.1042/BCJ20190463. Biochem J. 2020. PMID: 32059055 Review.
References
-
- Peng W., Shi L., Huang Q., Li T., Jian W., Zhao L., Xu R., Liu T., Zhang B., Wang H., et al. Metabolite profiles of distinct obesity phenotypes integrating impacts of altitude and their association with diet and metabolic disorders in tibetans. Sci. Total Environ. 2024;949:174754. doi: 10.1016/j.scitotenv.2024.174754. - DOI - PubMed
-
- Duca F., Mascherbauer K., Donà C., Koschutnik M., Binder C., Nitsche C., Halavina K., Beitzke D., Loewe C., Bartko P., et al. Association of epicardial adipose tissue on magnetic resonance imaging with cardiovascular outcomes: Quality over quantity? Obesity. 2024;32:1670–1679. doi: 10.1002/oby.24105. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

