Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue
- PMID: 40004089
- PMCID: PMC11855773
- DOI: 10.3390/ijms26041625
Platelets Modulate Leukocyte Population Composition Within Perivascular Adipose Tissue
Abstract
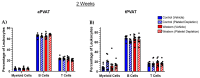
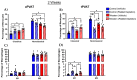
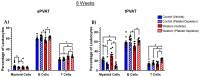
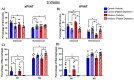
Perivascular adipose tissue (PVAT) regulates vascular tone and is composed of adipocytes and several leukocyte subpopulations. Diet can modify PVAT function, as obesogenic diets cause morphological changes to adipocytes and skew the leukocyte phenotype, leading to PVAT dysregulation and impaired vasoregulation. Of note, platelets, the clot-forming cells, also modulate many facets of leukocyte activity, such as tissue infiltration and polarity. We aimed to determine whether platelets regulate the leukocyte populations residing within PVAT. Male C57Bl/6J mice were fed a Western diet (30% kcal sucrose, 40% kcal fat, 8.0% sodium) to develop obesogenic conditions for PVAT leukocyte remodeling. Diet was either administered acutely (2 weeks) or extended (8 weeks) to gauge the length of challenge necessary for remodeling. Additionally, platelet depletion allowed for the assessment of platelet relevance in PVAT leukocyte remodeling. Abdominal PVAT (aPVAT) and thoracic PVAT (tPVAT) were then isolated and leukocyte composition evaluated by flow cytometry. Compared to control, Western diet alone did not significantly impact PVAT leukocyte composition for either diet length. Platelet depletion, independent of diet, significantly disrupted PVAT leukocyte content with monocytes/macrophages most impacted. Furthermore, tPVAT appeared more sensitive to platelet depletion than aPVAT, providing novel evidence of platelet regulation of leukocyte composition within PVAT depots.
Keywords: Western diet; leukocytes; macrophages; monocytes; obesity; perivascular adipose tissue; platelets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Ipragliflozin-induced adipose expansion inhibits cuff-induced vascular remodeling in mice.Cardiovasc Diabetol. 2019 Jun 24;18(1):83. doi: 10.1186/s12933-019-0886-1. Cardiovasc Diabetol. 2019. PMID: 31234839 Free PMC article.
-
Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice.Mol Metab. 2018 Mar;9:199-206. doi: 10.1016/j.molmet.2017.12.014. Epub 2018 Jan 10. Mol Metab. 2018. PMID: 29396370 Free PMC article.
-
Luseogliflozin attenuates neointimal hyperplasia after wire injury in high-fat diet-fed mice via inhibition of perivascular adipose tissue remodeling.Cardiovasc Diabetol. 2019 Oct 31;18(1):143. doi: 10.1186/s12933-019-0947-5. Cardiovasc Diabetol. 2019. PMID: 31672147 Free PMC article.
-
Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells.Arterioscler Thromb Vasc Biol. 2020 May;40(5):1094-1109. doi: 10.1161/ATVBAHA.120.312464. Epub 2020 Mar 19. Arterioscler Thromb Vasc Biol. 2020. PMID: 32188271 Free PMC article. Review.
-
Perivascular Adipose Tissue: the Sixth Man of the Cardiovascular System.Cardiovasc Drugs Ther. 2018 Oct;32(5):481-502. doi: 10.1007/s10557-018-6820-z. Cardiovasc Drugs Ther. 2018. PMID: 30171461 Free PMC article. Review.
References
-
- Gao Y.J. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr. Pharm. Des. 2007;13:2185–2192. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

