Comprehensive Bioinformatics Analysis of Glycosylation-Related Genes and Potential Therapeutic Targets in Colorectal Cancer
- PMID: 40004112
- PMCID: PMC11855181
- DOI: 10.3390/ijms26041648
Comprehensive Bioinformatics Analysis of Glycosylation-Related Genes and Potential Therapeutic Targets in Colorectal Cancer
Abstract
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide, characterized by high incidence and poor survival rates. Glycosylation, a fundamental post-translational modification, influences protein stability, signaling, and tumor progression, with aberrations implicated in immune evasion and metastasis. This study investigates the role of glycosylation-related genes (Glycosylation-RGs) in CRC using machine learning and bioinformatics. Data from The Cancer Genome Atlas (TCGA) and the Molecular Signatures Database (MSigDB) were analyzed to identify 67 differentially expressed Glycosylation-RGs. These genes were used to classify CRC patients into two subgroups with distinct survival outcomes, highlighting their prognostic value. Weighted gene coexpression network analysis (WGCNA) revealed key modules associated with CRC traits, including pathways like glycan biosynthesis and PI3K-Akt signaling. A machine-learning-based prognostic model demonstrated strong predictive performance, stratifying patients into high- and low-risk groups with significant survival differences. Additionally, the model revealed correlations between risk scores and immune cell infiltration, providing insights into the tumor immune microenvironment. Drug sensitivity analysis identified potential therapeutic agents, including Trametinib, SCH772984, and Oxaliplatin, showing differential efficacy between risk groups. These findings enhance our understanding of glycosylation in CRC, identifying it as a critical factor in disease progression and a promising target for future therapeutic strategies.
Keywords: bioinformatics; colorectal cancer; drug sensitivity analysis; glycosylation; immune microenvironment; machine learning; prognostic model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


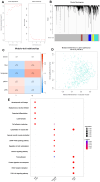

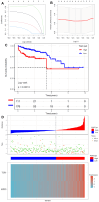

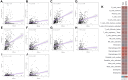

References
-
- Hossain M.S., Karuniawati H., Jairoun A.A., Urbi Z., Ooi D.J., John A., Lim Y.C., Kibria K.K., Mohiuddin A., Ming L.C. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers. 2022;14:1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

