Inhibitory Effects of Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) and Its Components on Cartilage Breakdown in Osteoarthritis
- PMID: 40004191
- PMCID: PMC11855050
- DOI: 10.3390/ijms26041728
Inhibitory Effects of Heat-Processed Gynostemma pentaphyllum Extract (Actiponin®) and Its Components on Cartilage Breakdown in Osteoarthritis
Abstract
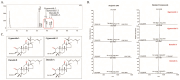
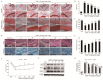
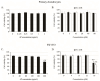
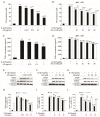
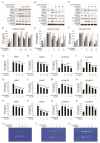
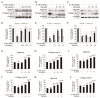
Osteoarthritis (OA), caused by the long-term use of joints, is a representative degenerative disease in the elderly. However, recently, the age of onset has been decreasing owing to excessive activities among young people in their 20s and 30s. Gynostemma pentaphyllum (Thunb.) Makino (GP), a perennial herb of the Cucurbitaceae family, has been used since the Ming dynasty as a medicinal material to treat various ailments, such as rheumatism, liver disease, and diabetes. In this study, we investigated the anti-arthritic effects of heat-processed Gynostemma pentaphyllum extract (Actiponin (AP)) and its derivatives, damulin A (DA) and damulin B (DB), using in vitro (primary rat chondrocytes and SW1353 cells) and in vivo (destabilization of the medial meniscus (DMM)-induced OA model) systems. Histological analysis results from the in vivo study showed that the group that underwent DMM surgery induced degeneration by the loss of proteoglycan and the destruction of cartilage (OARSI score 14 ± 0.57), whereas the group that received AP daily for 8 weeks maintained an intact condition (OARSI score 5 ± 0.28 at 200 mg/kg, p < 0.001). In addition, cartilage thickness and chondrocytes were reduced in the DMM group, but were restored in the AP-administered group. Furthermore, the von Frey analysis results showed that the pain threshold of the DMM group was considerably low (54.5 g at 8 weeks), whereas that of the AP group was dose-dependently increased (65.5, 69.5, 70.3, and 71.8 at 8 weeks for 30, 50, 100, and 200 mg/kg, respectively). In vitro studies showed that AP, DA, and DB reduced the expression of interleukin-1β alone-induced nitrite; inducible nitric oxide synthase; cyclooxygenase-2; matrix metallopeptidase 1/3/13; and a disintegrin and metalloproteinase with thrombospondin motifs 4/5. They also restored the expression of collagen type II and aggrecan, which are components of the extracellular matrix. The anti-arthritic effects of AP, DA, and DB were confirmed to be mediated by the mitogen-activated protein kinase and nuclear factor kappa-light-chain-enhancer of activated B cell signaling pathways. Collectively, these results suggest that AP is a potential therapeutic agent for mitigating OA progression and chondroprotection.
Keywords: Gynostemma pentaphyllum; actiponin; anti-arthritic; chondrocytes; osteoarthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

