Eosinophil-Driven vs. Eosinophil-Associated Severe Asthma: Practical Implications for Target Treatment
- PMID: 40004192
- PMCID: PMC11855446
- DOI: 10.3390/ijms26041729
Eosinophil-Driven vs. Eosinophil-Associated Severe Asthma: Practical Implications for Target Treatment
Abstract
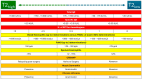
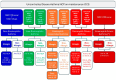
Severe asthma (SA) is a chronic inflammatory condition affecting approximately 10% of asthmatic patients, and eosinophils are considered key pathogenetic actors in a significant number of patients. Biological therapies have been demonstrated to improve asthma control by decreasing exacerbation rates and reducing the use of oral corticosteroids. In this context, phenotyping and endotyping patients with SA is essential for selecting the most effective therapeutic approach. For this purpose, biomarkers such as IgE, absolute blood eosinophil count, and fractional exhaled nitric oxide (FeNO) are crucial in defining a patient's inflammatory profile. Their integration provides a framework for classifying asthma into T2-high, T2-mild, or T2-low categories, guiding personalized treatment strategies. By incorporating multiple biomarkers into a unified model, it is possible to better stratify patients and optimize biologic therapy selection, paving the way for improved outcomes in SA management. This review aims to evaluate the role of phenotyping and endotyping SA patients, with particular attention to the impact of eosinophilic inflammation and combinatory biomarkers on decision-making processes for the selection of biological therapies.
Keywords: atopy biomarkers; biological therapies; eosinophils; personalized medicine; severe asthma; type 2 inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Association between pre-biologic T2-biomarker combinations and response to biologics in patients with severe asthma.Front Immunol. 2024 Apr 19;15:1361891. doi: 10.3389/fimmu.2024.1361891. eCollection 2024. Front Immunol. 2024. PMID: 38711495 Free PMC article.
-
Type 2 innate lymphoid cells: A novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma.Respir Med. 2015 Nov;109(11):1391-6. doi: 10.1016/j.rmed.2015.09.016. Epub 2015 Oct 9. Respir Med. 2015. PMID: 26459159
-
Type-2 airway inflammation in mild asthma patients with high blood eosinophils and high fractional exhaled nitric oxide.Clin Transl Sci. 2021 Jul;14(4):1259-1264. doi: 10.1111/cts.13078. Epub 2021 Jun 9. Clin Transl Sci. 2021. PMID: 34106513 Free PMC article.
-
How do biologicals and other novel therapies effect clinically used biomarkers in severe asthma?Clin Exp Allergy. 2020 Sep;50(9):994-1006. doi: 10.1111/cea.13694. Epub 2020 Jul 14. Clin Exp Allergy. 2020. PMID: 32569412 Review.
-
Exploring novel perspectives on eosinophilic inflammation in severe asthma.Biomark Med. 2024;18(8):357-361. doi: 10.2217/bmm-2023-0801. Epub 2024 Apr 16. Biomark Med. 2024. PMID: 38623926 Free PMC article. Review.
References
-
- Canonica G.W., Blasi F., Carpagnano G.E., Guida G., Heffler E., Paggiaro P., Allegrini C., Antonelli A., Aruanno A., Bacci E., et al. Severe Asthma Network Italy Definition of Clinical Remission in Severe Asthma: A Delphi Consensus. J. Allergy Clin. Immunol. Pract. 2023;11:3629–3637. doi: 10.1016/j.jaip.2023.07.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

