Application of an Integrated Single-Cell and Three-Dimensional Spheroid Culture Platform for Investigating Drug Resistance Heterogeneity and Epithelial-Mesenchymal Transition (EMT) in Lung Cancer Subclones
- PMID: 40004228
- PMCID: PMC11855057
- DOI: 10.3390/ijms26041766
Application of an Integrated Single-Cell and Three-Dimensional Spheroid Culture Platform for Investigating Drug Resistance Heterogeneity and Epithelial-Mesenchymal Transition (EMT) in Lung Cancer Subclones
Abstract
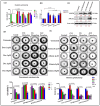
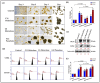
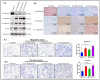
Lung cancer is a leading cause of cancer-related mortality worldwide, largely due to its heterogeneity and intrinsic drug resistance. Malignant pleural effusions (MPEs) provide diverse tumor cell populations ideal for studying these complexities. Although chemotherapy and targeted therapies can be initially effective, subpopulations of cancer cells with phenotypic plasticity often survive treatment, eventually developing resistance. Here, we integrated single-cell isolation and three-dimensional (3D) spheroid culture to dissect subclonal heterogeneity and drug responses, aiming to inform precision medicine approaches. Using A549 lung cancer cells, we established a cisplatin-resistant line and isolated three resistant subclones (Holoclone, Meroclone, Paraclone) via single-cell sorting. In 3D spheroids, Docetaxel and Alimta displayed higher IC50 values than in 2D cultures, suggesting that 3D models better reflect clinical dosing. Additionally, MPE-derived Holoclone and Paraclone subclones exhibited distinct sensitivities to Giotrif and Capmatinib, revealing their heterogeneous drug responses. Molecular analyses confirmed elevated ABCB1, ABCG2, cancer stem cell (CSC) markers (OCT4, SOX2, CD44, CD133), and epithelial-mesenchymal transition (EMT) markers (E-cadherin downregulation, increased Vimentin, N-cadherin, Twist) in resistant subclones, correlating with enhanced migration and invasion. This integrated approach clarifies the interplay between heterogeneity, CSC/EMT phenotypes, and drug resistance, providing a valuable tool for predicting therapeutic responses and guiding personalized, combination-based lung cancer treatments.
Keywords: cancer stem cells; drug resistance; epithelial–mesenchymal transition; lung cancer; single-cell culture; targeted therapy; three-dimensional spheroid culture.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bhat G.R., Sethi I., Sadida H.Q., Rah B., Mir R., Algehainy N., Albalawi I.A., Masoodi T., Subbaraj G.K., Jamal F., et al. Cancer cell plasticity: From cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024;43:197–228. doi: 10.1007/s10555-024-10172-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

