Mechanism of Action and Interaction of Garlic Extract and Established Therapeutics in Prostate Cancer
- PMID: 40004239
- PMCID: PMC11855885
- DOI: 10.3390/ijms26041777
Mechanism of Action and Interaction of Garlic Extract and Established Therapeutics in Prostate Cancer
Abstract
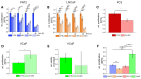
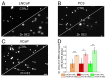
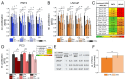
A detailed characterization of the mechanism of action of garlic extract (GE) on prostate cancer (PCa) cells is essential to ensure its safe use as a complementary therapy, particularly when combined with established treatments. A case report highlighted the potential benefits of GE in PCa management. A patient diagnosed with PCa, presenting an initial prostate-specific antigen (PSA) of 11.8 ng/mL, maintained PSA levels between 3.5 and 6 ng/mL for over 14 years with daily GE intake. To study GE's anti-proliferative effects and interactions with established therapeutics, healthy prostate epithelial cells (PNT2) and PCa cells (LNCaP, PC3, VCaP) were treated with GE. Proliferation, Integrin β1 pattern, DNA-damage, as well as androgen receptor (AR) and Cytochrome P450 (CYP450) expression were investigated. GE reduced the proliferation of LNCaP and PC3 cells compared to healthy PNT2 cells but had contrary effects on VCaP cells. The combination of GE with standard therapies, including chemotherapy, androgen deprivation therapy (ADT), and Poly-(ADP-ribose)-Polymerase inhibitors (PARPi), reduced the efficacy of these treatments in tumor cells, potentially due to the GE-induced upregulation of the metabolic enzyme CYP2C9 in PCa cell lines. These findings indicate that while GE has anti-proliferative effects, the use of highly concentrated natural extracts must be carefully assessed by expert physicians on a case-by-case basis, especially when combined with established therapies.
Keywords: CYP450; androgen receptor; drug-interaction; garlic; natural products; prostate cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Oliva J., Anastay V., Baboudjian M., Roumiguie M., Peltier A., Dariane C., Fiard G., Roumeguere T., Diamand R., Bakhri M., et al. Active surveillance for low-risk prostate cancer with high tumor burden at biopsy: Lessons learned from a contemporary radical prostatectomy cohort. World J. Urol. 2024;42:513. doi: 10.1007/s00345-024-05227-3. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

