The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells
- PMID: 40004517
- PMCID: PMC11855201
- DOI: 10.3390/genes16020188
The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells
Abstract
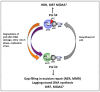
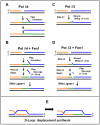
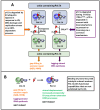
There are two forms of DNA polymerase δ in human cells, Pol δ4 and Pol δ3, which differ based on their possession of the p12 subunit. The degradation of p12 has emerged as an important regulatory mechanism that controls the generation of Pol δ3. The underlying importance of this system lies in the altered enzymatic properties of the two forms of Pol δ engendered by the influence of p12. We briefly review how the balance of these two forms is regulated through the degradation of p12. We focus on the roles of Pol δ4, whose cellular functions are less well known. This is significant because recent studies show that this is the form engaged in the homology-dependent repair of double-strand breaks. We consider new horizons for future research into this system and their potential involvement in tumorigenesis.
Keywords: DNA damage response; DNA polymerase δ; DNA repair; cell cycle regulation; homology-directed repair; human DNA replication; tumorigenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The tail that wags the dog: p12, the smallest subunit of DNA polymerase δ, is degraded by ubiquitin ligases in response to DNA damage and during cell cycle progression.Cell Cycle. 2014;13(1):23-31. doi: 10.4161/cc.27407. Epub 2013 Dec 3. Cell Cycle. 2014. PMID: 24300032 Free PMC article.
-
Loss of the p12 subunit of DNA polymerase delta leads to a defect in HR and sensitization to PARP inhibitors.DNA Repair (Amst). 2019 Jan;73:64-70. doi: 10.1016/j.dnarep.2018.11.003. Epub 2018 Nov 13. DNA Repair (Amst). 2019. PMID: 30470508 Free PMC article.
-
A novel function of CRL4(Cdt2): regulation of the subunit structure of DNA polymerase δ in response to DNA damage and during the S phase.J Biol Chem. 2013 Oct 11;288(41):29550-61. doi: 10.1074/jbc.M113.490466. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913683 Free PMC article.
-
Two forms of human DNA polymerase δ: Who does what and why?DNA Repair (Amst). 2019 Sep;81:102656. doi: 10.1016/j.dnarep.2019.102656. Epub 2019 Jul 8. DNA Repair (Amst). 2019. PMID: 31326365 Review.
-
Multiple Forms of Human DNA Polymerase Delta Sub-Assembling in Cellular DNA Transactions.Curr Protein Pept Sci. 2016;17(8):746-755. doi: 10.2174/1389203717666160226145006. Curr Protein Pept Sci. 2016. PMID: 26916162 Review.
Cited by
-
Beyond proofreading: POLD1 mutations as dynamic orchestrators of genomic instability and immune evasion in cancer.Front Immunol. 2025 Jun 30;16:1600233. doi: 10.3389/fimmu.2025.1600233. eCollection 2025. Front Immunol. 2025. PMID: 40661943 Free PMC article. Review.
References
-
- Lee M.Y., Zhang S., Lin S.H., Wang X., Darzynkiewicz Z., Zhang Z., Lee E.Y. The tail that wags the dog: p12, the smallest subunit of DNA polymerase δ, is degraded by ubiquitin ligases in response to DNA damage and during cell cycle progression. Cell Cycle. 2014;13:23–31. doi: 10.4161/cc.27407. - DOI - PMC - PubMed
-
- Zhang S., Zhou Y., Sarkeshik A., Yates J.R., 3rd, Thomson T.M., Zhang Z., Lee E.Y., Lee M.Y. Identification of RNF8 as a ubiquitin ligase involved in targeting the p12 subunit of DNA polymerase δ for degradation in response to DNA damage. J. Biol. Chem. 2013;288:2941–2950. doi: 10.1074/jbc.M112.423392. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

