Molecular Review of Suspected Alport Syndrome Patients-A Single-Centre Experience
- PMID: 40004525
- PMCID: PMC11855357
- DOI: 10.3390/genes16020196
Molecular Review of Suspected Alport Syndrome Patients-A Single-Centre Experience
Abstract
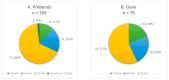
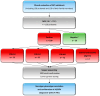
Background: Alport syndrome (AS) is a clinically and genetically heterogeneous glomerulopathy resulting from pathogenic variants in COL4A3, COL4A4, and COL4A5. Genetic diagnosis is increasingly being conducted using next-generation sequencing (NGS). Methods: Within eight years, we examined a group of 247 Polish individuals and found in total 138 unrelated probands suspected with AS based on clinical course, laboratory findings, and/or family history, as well as the total of 109 family members. We applied a targeted NGS panel to identify the genetic spectrum of AS. Known and novel variants were revealed, and detailed evaluation was performed according to ACMG/AMP guidelines to classify them as pathogenic/likely pathogenic/VUS changes. Identified genotypes were compared with clinical manifestations: hematuria, proteinuria, chronic kidney disease, sensorineural hearing impairment, ocular abnormalities, and hypertension. Results: The molecular background was established in 109/138 probands. Overall, 79 different COL4A3-COL4A5 changes (56 known and 23 novel) were revealed. About 97% were SNVs, and only two COL4A5 CNVs were identified. In total, 11 recurrent COL4A3-COL4A5 variants were observed, including the most frequent COL4A5:p.Gly624Asp, accounting for 31% of X-linked AS. Conclusions: The use of NGS panel has shown considerable promise in the field of AS, increasing diagnostic rate to 79% and reducing time to diagnosis. The phenotype-driven gene panel, specific for genetic diseases in the pediatric population, is an affordable alternative to WGS and WES, offering comparable diagnostic efficacy and supporting its implementation as a first-line genetic test in rare diseases, including AS. Based on the obtained genotype-phenotype correlation, we assessed that NGS allows us to avoid invasive renal biopsy in AS diagnosis. It provides AS confirmation/exclusion, atypical AS identification, symptomatic/asymptomatic monoallelic COL4A3-COL4A5 carrier (especially COL4A5 females) determination, and inheritance pattern establishment. AS diagnosis confirmation enables clinical course prediction and is crucial for the early introduction of renoprotective treatment with renin-angiotensin-aldosterone system blockade, aimed at slowing the disease progression and estimating the risk in family members, which is important for genetic counselling.
Keywords: Alport syndrome; COL4A3; COL4A4; COL4A5; genetic testing; next-generation sequencing; novel molecular variants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Savige J., Storey H., Watson E., Hertz J.M., Deltas C., Renieri A., Mari F., Hilbert P., Plevova P., Byers P., et al. Consensus Statement on Standards and Guidelines for the Molecular Diagnostics of Alport Syndrome: Refining the ACMG Criteria. Eur. J. Hum. Genet. 2021;29:1186–1197. doi: 10.1038/s41431-021-00858-1. - DOI - PMC - PubMed
-
- Gibson J., Fieldhouse R., Chan M.M.Y., Sadeghi-Alavijeh O., Burnett L., Izzi V., Persikov A.V., Gale D.P., Storey H., Savige J., et al. Prevalence Estimates of Predicted Pathogenic COL4A3-COL4A5 Variants in a Population Sequencing Database and Their Implications for Alport Syndrome. J. Am. Soc. Nephrol. 2021;32:2273–2290. doi: 10.1681/ASN.2020071065. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

