Real-World Effectiveness, Safety, and Tolerability of Facilitated Subcutaneous Immunoglobulin 10% in Secondary Immunodeficiency Disease: A Systematic Literature Review
- PMID: 40004732
- PMCID: PMC11856383
- DOI: 10.3390/jcm14041203
Real-World Effectiveness, Safety, and Tolerability of Facilitated Subcutaneous Immunoglobulin 10% in Secondary Immunodeficiency Disease: A Systematic Literature Review
Abstract
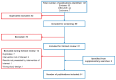
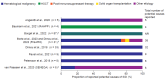
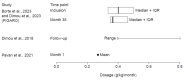
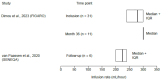
Background: Secondary immunodeficiency disease (SID) is a complex, heterogeneous condition that occurs when extrinsic factors weaken the immune system. Expert consensus guidelines recommend immunoglobulin replacement therapy to manage immunoglobulin G (IgG) levels and mitigate severe, recurrent, and persistent infections. Hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) 10% is a dual-vial unit of IgG and recombinant human hyaluronidase; the latter enables absorption of higher volumes of IgG than conventional subcutaneous therapies. Methods: For this systematic literature review, Embase, MEDLINE®, and the Cochrane Library were searched on 9 August 2023, with supplemental congress searches. Results: Eight studies fulfilled the inclusion criteria, reporting real-world evidence of the clinical effectiveness, safety, and tolerability of fSCIG 10% in 183 patients with SID in Europe from September 2014 to August 2021. The potential causes of SID were primarily hematological malignancies, most commonly chronic lymphocytic leukemia. Treatment was typically administered at 4-week or 3-week intervals, with doses of approximately 0.4 g/kg/month. Infections were rare during follow-up, with numerical reductions observed after fSCIG 10% treatment initiation compared with the period before initiation. Adverse reactions, including local infusion site reactions, and tolerability events were uncommon. Conclusions: Given the recency of fSCIG 10% use in patients with SID, there are opportunities for future research to better understand survival and patient-reported outcomes after receiving this treatment. Despite SID heterogeneity, this study demonstrates the feasibility of fSCIG 10% treatment for this condition.
Keywords: hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) 10%; infection; real-world evidence; safety; secondary immunodeficiency; tolerability.
Conflict of interest statement
M.D., S.S.-R. and E.K.-P. have no conflicts of interest to disclose. A.V. has received access to scientific resources and protected time for conducting this research from funding granted by the “PIANO NAZIONALE DI RIPRESA E RESILIENZA—PNRR” mission 4—Component C2, “Fondo per il Programma Nazionale di Ricerca e Progetti di Rilevante Interesse Nazionale—PRIN” (n.2022ZKKWLW), and from the Ministero della Salute-Progetto “AmICA: Assistenza olistica Intelligente per l’aCtive Ageing in ecosistemi indoor e outdoor”, Traiettoria 1 “Active & Healthy Ageing—Tecnologie per l’invecchiamento attivo e l’assistenza domiciliare”(T1-MZ-09). V.L. is an employee of Oxford PharmaGenesis Ltd., Oxford, UK, which was contracted by Takeda Pharmaceuticals International AG to support the design and conduct of the systematic literature review. C.S. and M.K. are employees of Takeda Development Center Americas, Inc. and are Takeda shareholders. C.A.-S. was an employee of Takeda Development Center Americas, Inc. and a Takeda shareholder at the time of the study, and is now an employee of Gilead Sciences.
Figures

References
-
- Rider N.L., Truxton A., Ohrt T., Margolin-Katz I., Horan M., Shin H., Davila R., Tenembaum V., Quinn J., Modell V., et al. Validating inborn error of immunity prevalence and risk with nationally representative electronic health record data. J. Allergy Clin. Immunol. 2024;153:1704–1710. doi: 10.1016/j.jaci.2024.01.011. - DOI - PubMed
-
- Ochoa-Grullon J., Guevara-Hoyer K., Perez Lopez C., Perez de Diego R., Pena Cortijo A., Polo M., Mateo Morales M., Anguita Mandley E., Jimenez Garcia C., Bolanos E., et al. Combined immune defect in B-cell lymphoproliferative disorders is associated with severe infection and cancer progression. Biomedicines. 2022;10:2020. doi: 10.3390/biomedicines10082020. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

