Use of Primary Prophylaxis with G-CSF in Acute Myeloid Leukemia Patients Undergoing Intensive Chemotherapy Does Not Affect Quality of Response
- PMID: 40004785
- PMCID: PMC11856925
- DOI: 10.3390/jcm14041254
Use of Primary Prophylaxis with G-CSF in Acute Myeloid Leukemia Patients Undergoing Intensive Chemotherapy Does Not Affect Quality of Response
Abstract
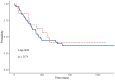
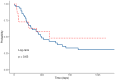
Background/Objectives: The objective of our study was to evaluate the safety and efficacy of granulocyte colony-stimulating factor (G-CSF) as primary prophylaxis in adult patients with acute myeloid leukemia (AML) undergoing intensive chemotherapy. Methods: We retrospectively analyzed 112 AML patients treated with intensive chemotherapy at Fondazione Policlinico Tor Vergata in Rome between January 2014 and March 2024. Patients were divided into G-CSF and non-G-CSF (nG-CSF) groups. We assessed the incidence of neutropenia, its severity and duration; duration of hospitalization and its costs; incidence of febrile neutropenia (FN) and septic shock; duration of antibiotic therapy (ABT) and antifungal therapy (AFT); complete remission (CR) rates; measurable residual disease (MRD) status; relapse rates; and outcomes. Results: G-CSF administration significantly reduced the duration of neutropenia (median 14 vs. 18 days, p < 0.05) and length of hospitalization (median 28 vs. 35 days, p < 0.05), in both induction and consolidation therapy. There were no significant differences in CR rates (73% vs. 67%, p = 0.64), MRD negativity achievement (52% vs. 48%, p = 0.68), leukemia relapse rates (43% vs. 62%, p = 0.14), or overall survival (OS) (median 16.7 vs. 12.3 months, p = 0.3) between G-CSF and nG-CSF groups. Thanks to a shorter hospitalization, the use of G-CSF led to €300,000 in savings over the last 4 years. Conclusions: Our findings support the safety of G-CSF in AML patients, demonstrating no adverse impact on treatment response. G-CSF abbreviated the duration of neutropenia and hospitalization, highlighting its potential clinical and cost-effective role in AML treatment.
Keywords: acute myeloid leukemia; febrile neutropenia; granulocyte colony-stimulating factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Neutropenia-associated outcomes in adults with acute myeloid leukemia receiving cytarabine consolidation chemotherapy with or without granulocyte colony-stimulating factor.Pharmacotherapy. 2012 Dec;32(12):1070-7. doi: 10.1002/phar.1150. Pharmacotherapy. 2012. PMID: 23208834
-
Granulocyte colony-stimulating factor after intensive consolidation chemotherapy in acute myeloid leukemia: results of a randomized trial of the Groupe Ouest-Est Leucémies Aigues Myeloblastiques.J Clin Oncol. 2000 Feb;18(4):780-7. doi: 10.1200/JCO.2000.18.4.780. J Clin Oncol. 2000. PMID: 10673519 Clinical Trial.
-
[A Real-World Study of the Effect of rhG-CSF on Clinical Efficacy and Flow Cytometry MRD after Initial Induction Therapy for Acute Myeloid Leukemia].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022 Aug;30(4):1022-1027. doi: 10.19746/j.cnki.issn.1009-2137.2022.04.008. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022. PMID: 35981357 Chinese.
-
Impact on acute myeloid leukemia relapse in granulocyte colony-stimulating factor application: a meta-analysis.Hematology. 2018 Oct;23(9):581-589. doi: 10.1080/10245332.2018.1446811. Epub 2018 Mar 8. Hematology. 2018. PMID: 29516766 Review.
-
Effectiveness and safety of primary prophylaxis with G-CSF after induction therapy for acute myeloid leukemia: a systematic review and meta-analysis of the clinical practice guidelines for the use of G-CSF 2022 from the Japan society of clinical oncology.Int J Clin Oncol. 2024 May;29(5):535-544. doi: 10.1007/s10147-023-02465-0. Epub 2024 Mar 18. Int J Clin Oncol. 2024. PMID: 38494578 Free PMC article.
References
-
- Pandya B.J., Chen C.-C., Medeiros B.C., McGuiness C.B., Wilson S.D., Walsh E.H., Wade R.L. Economic and Clinical Burden of Acute Myeloid Leukemia Episodes of Care in the United States: A Retrospective Analysis of a Commercial Payer Database. J. Manag. Care Spec. Pharm. 2020;26:849–859. doi: 10.18553/jmcp.2020.19220. - DOI - PMC - PubMed
-
- Döhner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. - DOI - PubMed
-
- Acute Myeloid Leukemia—Cancer Stat Facts. SEER. [(accessed on 20 January 2025)]; Available online: https://seer.cancer.gov/statfacts/html/amyl.html.
-
- Lambert J., Pautas C., Terré C., Raffoux E., Turlure P., Caillot D., Legrand O., Thomas X., Gardin C., Gogat-Marchant K., et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104:113–119. doi: 10.3324/haematol.2018.188888. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous