Unveiling the Therapeutic Potential of the Second-Generation Incretin Analogs Semaglutide and Tirzepatide in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults
- PMID: 40004833
- PMCID: PMC11856673
- DOI: 10.3390/jcm14041303
Unveiling the Therapeutic Potential of the Second-Generation Incretin Analogs Semaglutide and Tirzepatide in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults
Abstract
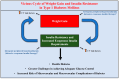
Type 1 diabetes mellitus (T1D) is a chronic autoimmune disease caused by the immune-mediated destruction of insulin-producing pancreatic beta cells, resulting in the lifelong need for exogenous insulin. Over the last few years, overweight and obesity have recently emerged as growing health issues also afflicting patients with T1D. In this context, the term "double diabetes" has been coined to indicate patients with T1D who have a family history of type 2 diabetes mellitus (T2D) and/or patients with T1D who are affected by insulin resistance and/or overweight/obesity and/or metabolic syndrome. At the same time, the use of second-generation incretin analogs semaglutide and tirzepatide has substantially increased on a global scale over the last few years, given the remarkable clinical benefits of these drugs (in terms of glucose control and weight loss) in patients with T2D and/or overweight/obesity. Although the glucagon-like peptide-1 (GLP-1) receptor agonists and the novel dual GIP (glucose-dependent insulinotropic polypeptide)/GLP-1 receptor agonist tirzepatide are currently not approved for the treatment of T1D, a growing body of evidence over the last few years has shown that these medications may serve as valid add-on treatments to insulin with substantial efficacy in improving glucose control, promoting weight loss, preserving residual beta-cell function and providing other beneficial metabolic effects in patients with T1D, double diabetes and latent autoimmune diabetes in adults (LADA). This manuscript aims to comprehensively review the currently available literature (mostly consisting of real-world studies) regarding the safety and therapeutic use (for different purposes) of semaglutide and tirzepatide in patients with T1D (at different stages of the disease), double diabetes and LADA.
Keywords: C-peptide; GIP; GLP-1; LADA; T1D; autoimmune diabetes; beta-cell function; double diabetes; obesity; overweight; semaglutide; tirzepatide; type 1 diabetes.
Conflict of interest statement
CR serves as a Scientific Advisor for Novo Nordisk® (Bagsværd, Denmark). The other authors declare no conflicts of interest.
Figures

References
-
- Perkovic V., Tuttle K.R., Rossing P., Mahaffey K.W., Mann J.F.E., Bakris G., Baeres F.M.M., Idorn T., Bosch-Traberg H., Lausvig N.L., et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024;391:109–121. doi: 10.1056/NEJMoa2403347. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

