Temporal Trends in the Use of Biological Agents in Patients with Inflammatory Bowel Disease: Real-World Data from a Tertiary Inflammatory Bowel Disease Greek Center During a 5-Year Period
- PMID: 40004889
- PMCID: PMC11856159
- DOI: 10.3390/jcm14041357
Temporal Trends in the Use of Biological Agents in Patients with Inflammatory Bowel Disease: Real-World Data from a Tertiary Inflammatory Bowel Disease Greek Center During a 5-Year Period
Abstract
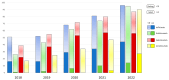
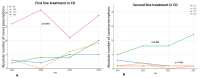
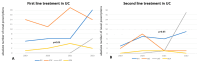
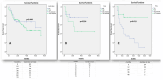
Background/Objectives: Therapeutic management of inflammatory bowel diseases (IBD) is rapidly evolving in the era of novel biological therapies. However, real-world data relating to the usage trends and treatment persistence remain inconsistent. This study aimed to investigate trends in biological use, dose intensification, and treatment persistence in IBD patients, who received treatment in a large tertiary center in Greece. Methods: Patients with IBD who underwent at least one biological treatment between 2018 and 2022 were included in this retrospective study. Data on patients' demographics, type of disease, use of biologicals, dose intensification, and treatment persistence were analyzed for time trends. Results: Data from 409 patients with IBD (mean age 39 (range 17-87), female 51%, 56.9% CD, mean duration of disease: 9.3 years) were included in the study. The number of patients on biologics was raised from 133 in 2018 to 368 in 2022 (a 28.1% yearly increase), while the percentage of patients who were treated with anti-TNF biosimilars increased to >60% of the total anti-TNF population in 2022. We observed a gradual increase in non-anti-TNF therapies in bio-naïve patients, in particular vedolizumab (46% of all biologicals in UC; 16% in CD) and ustekinumab (16.3% of all biologicals in UC, 31% in CD). The 3-year persistence rate of IFX was 64% in CD and 56% in UC, whereas it was 61% for ADA in CD. Dose intensification of anti-TNF was efficient in >50% of CD patients and >30% of UC patients; however, the majority of patients who required dose escalation within the first year eventually became unresponsive. The 3-year persistence of vedolizumab as a first-line treatment was 82% for CD and 69% for UC, respectively. The 3-year persistence of ustekinumab as first-line treatment for CD was 65%. No significant differences regarding the efficacy of anti-TNF, ustekinumab, or vedolizumab were detected when they were used as first-line treatments for Crohn's disease; similarly, no significant differences were detected between infliximab and vedolizumab as first-line treatments for UC. Conclusions: There was a gradual increase in the use of biologicals, including biosimilars, between the years 2018-2022, reflecting adherence to current guidance with adoption of an early escalation strategy. Newer, post-anti-TNF biologics such as vedolizumab and ustekinumab have been rapidly incorporated into therapeutic approaches for both CD and UC.
Keywords: biologicals; dose intensification; inflammatory bowel disease; time trends; treatment persistence.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: G.B. has received honoraria as an advisor/lecturer and/or research grants and/or clinical trial participant from AbbVie, Adacyte Therapeutics, Aenorasis, Amgen, Bristol Myers Squibb, Cooper, Ferring, Genesis Pharma, MSD, Mylan/Viatris, Janssen, Pfizer and Takeda; The rest of the authors declare no conflicts of interest.
Figures

Similar articles
-
10 years of biologic use patterns in patients with inflammatory bowel disease: treatment persistence, switching and dose intensification - a nationwide population-based study.Therap Adv Gastroenterol. 2023 Sep 29;16:17562848231201728. doi: 10.1177/17562848231201728. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37786473 Free PMC article.
-
Superior treatment persistence with ustekinumab in Crohn's disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study.Aliment Pharmacol Ther. 2021 Aug;54(3):292-301. doi: 10.1111/apt.16436. Epub 2021 Jun 20. Aliment Pharmacol Ther. 2021. PMID: 34151447
-
Superior persistence of ustekinumab compared to anti-TNF in vedolizumab-experienced inflammatory bowel diseases patients: a real-world cohort study.BMC Gastroenterol. 2024 Dec 31;24(1):483. doi: 10.1186/s12876-024-03577-1. BMC Gastroenterol. 2024. PMID: 39741232 Free PMC article.
-
Biologics versus Immunomodulators for the Treatment of Ulcerative Colitis: A Review of Comparative Clinical Effectiveness and Cost-Effectiveness [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Apr 17. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Apr 17. PMID: 31693324 Free Books & Documents. Review.
-
Safety and Efficacy of Biologic Therapies (Ustekinumab and Vedolizumab) in the Treatment of Inflammatory Bowel Disease (IBD): A Systematic Review.Cureus. 2023 Nov 6;15(11):e48338. doi: 10.7759/cureus.48338. eCollection 2023 Nov. Cureus. 2023. PMID: 38060699 Free PMC article. Review.
References
-
- Markopoulos P., Karmiris K., Dimas I., Voudoukis E., Siakavellas S., Axiaris G., Zacharopoulou E., Zampeli E., Tsironi E., Tzouvala M., et al. Efficacy of Vaccination and Revaccination Against Hepatitis B Virus Using 2 Different Strategies in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024:izae173. doi: 10.1093/ibd/izae173. - DOI - PubMed
-
- Krugliak Cleveland N., Ghosh S., Chastek B., Bancroft T., Candela N., Fan T., Umashankar K., Rubin D.T. Real-World Persistence of Successive Biologics in Patients with Inflammatory Bowel Disease: Findings From ROTARY. Inflamm. Bowel Dis. 2023;30:izad245. doi: 10.1093/ibd/izad245. - DOI - PMC - PubMed
-
- Feuerstein J.D., Isaacs K.L., Schneider Y., Siddique S.M., Falck-Ytter Y., Singh S., Chachu K., Day L., Lebwohl B., Muniraj T., et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158:1450–1461. doi: 10.1053/j.gastro.2020.01.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

