Sequence of Eating at Japanese-Style Set Meals Improves Postprandial Glycemic Elevation in Healthy People
- PMID: 40004986
- PMCID: PMC11858527
- DOI: 10.3390/nu17040658
Sequence of Eating at Japanese-Style Set Meals Improves Postprandial Glycemic Elevation in Healthy People
Abstract
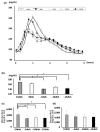
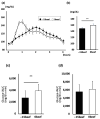
Background: The meal sequencing of macronutrients has been shown to ameliorate postprandial glucose excursion, but its effects in daily meals has not been investigated. We examined the impact on the glucose response to meal sequencing in healthy Japanese adults using continuous glucose monitoring (CGM) during a typical lunch meal.
Methods: The test meal was a Japanese set meal or a beef and rice bowl, the contents of which were categorized as "rice" or "non-rice". In the meal experiments, the subjects ingested the two categories of food in one of three orders: non-rice before rice, non-rice and rice together, and non-rice after rice. In the beef and rice bowl experiments, the subjects ingested either non-rice 15 min before rice or the two foods together.
Results: The postprandial glucose level was measured over a 4 h period and the mean level of postprandial glucose was significantly lower than that when eating rice before non-rice or both together. Consuming non-rice before rice significantly reduced postprandial glycemic excursions in healthy adults in both experiments.
Conclusions: Meal-sequencing by "eat carbs last" is a feasible dietary strategy for the better prevention and management of diabetes.
Keywords: diabetes; diet therapy; glucose monitoring; incretin; meal sequence.
Conflict of interest statement
Y.Y. (Yuji Yamazaki) received honoraria for lectures from Sumitomo Pharma Co., Mitsubishi Tanabe Pharma Corporation, and Eli Lilly Japan K.K. H.K. received grants from Ono Pharmaceutical Co., Taisho Pharmaceutical Co. and Novo Nordisk Pharma Ltd.; and honoraria for lectures from Sanofi K.K. and Taisho Pharmaceutical Co. Y.H. received grants from Sumitomo Pharma Co. and Nippon Boehringer Ingelheim Co., Ltd.; and honoraria for lectures from Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, Taisho Pharmaceutical Co., MSD K.K. and Sumitomo Pharma Co. Y.S. has received grants from Nippon Boehringer Ingelheim Co., Ltd., ARKRAY Marketing, Inc., Taisho Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Terumo Corporation and Sumitomo Pharma Co.; and honoraria for lectures from Taisho Pharmaceutical Co., Ltd., Nippon Becton Dickinson Company, Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Sumitomo Pharma Co., Ltd. and Ono Pharmaceutical Co., Ltd. Y.Y. (Yuichiro Yamada) has received honoraria for lectures from Ono Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Novo Nordisk Pharma Ltd. and Teijin Pharma Limited. The other authors declare no conflicts of interest.
Figures
References
-
- Okada A., Kaneko H., Matsuoka S., Itoh H., Suzuki Y., Fujiu K., Michihata N., Jo T., Takeda N., Morita H., et al. Association of cardiovascular health metrics with annual incidence of prediabetes or diabetes: Analysis of a nationwide real-world database. J. Diabetes Investig. 2023;14:452–462. doi: 10.1111/jdi.13958. - DOI - PMC - PubMed
-
- Ceriello A., Esposito K., Piconi L., Ihnat M.A., Thorpe J.E., Testa R., Boemi M., Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

