Preliminary Data on the Senolytic Effects of Agrimonia pilosa Ledeb. Extract Containing Agrimols for Immunosenescence in Middle-Aged Humans: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Study
- PMID: 40004995
- PMCID: PMC11858573
- DOI: 10.3390/nu17040667
Preliminary Data on the Senolytic Effects of Agrimonia pilosa Ledeb. Extract Containing Agrimols for Immunosenescence in Middle-Aged Humans: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Study
Abstract
Objectives: To assess the effects of agrimol-containing Agrimonia pilosa Ledeb. extract (APE) for senescent immune cell removal in middle-aged Japanese adults with immunosenescence.
Design and setting: A randomized, double-blind, placebo-controlled, parallel-group study was conducted in Japan between June 2023 and April 2024.
Participants: 110 individuals aged 40-59, selected based on CD8+ T cells with highly-expressing-senescence-associated-β-galactosidase (SA-βGal).
Intervention: Participants were randomly assigned to receive 50 mg APE containing 0.2 mg of agrimols or a placebo for eight consecutive weeks.
Measurements: The primary endpoint was the change in the proportion of CD8+ T cells with high SA-βGal expression at 8 weeks of intake from the baseline. The secondary endpoints included the proportion of CD4+ T cells with high SA-βGal expression, CD4+ and CD8+ T cell subsets, and the ratio of various immune cells.
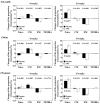
Results: Of the 635 subjects screened, 110 with immunosenescence were included in this study. In total, 55 participants in the placebo group and 53 in the APE group completed the intervention. There were no statistically significant changes in either the primary or secondary endpoints due to APE intake. In the male population, the proportion of CD8+ T cells with high SA-βGal expression was reduced by APE intake (p = 0.044). Furthermore, the proportion of naïve CD8+ T cells increased and the number of effector memory CD8+ T cells decreased with the consumption of APE.
Conclusions: APE was suggested to reduce senescent immune cells, indicating its potential as a candidate senolytic agent for humans; however, the results of this study are preliminary data, and further research on APE is needed (clinical trial registration: UMIN000051574).
Keywords: Agrimonia pilosa Ledeb. extract; agrimol; immunosenescence; senescence-associated β-galactosidase; senolytic agent.
Conflict of interest statement
Y.S., S.S., M.H., M.Y., T.S., T.W., and Y.I. are employees of FANCL. S.T. is an officer at FANCL. FANCL has applied for a patent for
Figures

References
-
- World Population Prospects 2022: Summary of Results. [(accessed on 10 May 2024)]. Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospect....
-
- Beard J.R., Officer A., de Carvalho I.A., Sadana R., Pot A.M., Michel J.P., Lloyd-Sherlock P., Epping-Jordan J.E., Peeters G., Mahanani W.R., et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

