Pleurotus eryngii Mushrooms Fermented with Human Fecal Microbiota Protect Intestinal Barrier Integrity: Immune Modulation and Signalling Pathways Counter Deoxycholic Acid-Induced Disruption in Healthy Colonic Tissue
- PMID: 40005021
- PMCID: PMC11858169
- DOI: 10.3390/nu17040694
Pleurotus eryngii Mushrooms Fermented with Human Fecal Microbiota Protect Intestinal Barrier Integrity: Immune Modulation and Signalling Pathways Counter Deoxycholic Acid-Induced Disruption in Healthy Colonic Tissue
Abstract
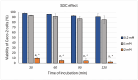
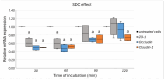
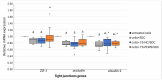
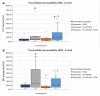
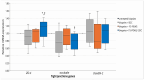
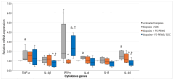
Background: This study explores the potential of the Pleurotus eryngii mushroom fermentation supernatant (FS-PEWS) as an intervention for mitigating sodium deoxycholate (SDC)-induced intestinal barrier dysfunction and inflammation. Methods: FS-PEWS was assessed for its protective effects against SDC-induced barrier dysfunction and inflammation using an in vitro Caco-2 cell model and ex vivo colonic biopsies from healthy adult donors, where barrier integrity, permeability, immunomodulation and receptor-mediated pathways were evaluated. Results: In Caco-2 cells, SDC exposure downregulated ZO-1, occludin, and claudin-1 expression, with FS-PEWS restoring ZO-1 and claudin-1 levels while maintaining cell viability. In colonic biopsies from healthy adults, FS-PEWS maintained tissue integrity and selectively mitigated transcellular permeability without affecting paracellular permeability when combined with the stressor. Additionally, FS-PEWS exhibited potent anti-inflammatory effects, reducing pro-inflammatory cytokines, e.g., TNF-α, IL-6, and IL-1β and modulating receptor-mediated pathways, i.e., TLR-4, dectin-1. Conclusions: These results demonstrate the potential of FS-PEWS to sustain intestinal barrier function and modulate immune responses under stress, highlighting its therapeutic potential for managing gut barrier dysfunction and inflammation associated with microbial metabolite-induced disruptions.
Keywords: Pleurotus eryngii mushrooms; Ussing chamber; cytokines; deoxycholic bile acid; gut barrier; signalling pathway.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

