Dietary Fatty Acid Composition Alters Gut Microbiome in Mice with Obesity-Induced Peripheral Neuropathy
- PMID: 40005065
- PMCID: PMC11858455
- DOI: 10.3390/nu17040737
Dietary Fatty Acid Composition Alters Gut Microbiome in Mice with Obesity-Induced Peripheral Neuropathy
Abstract
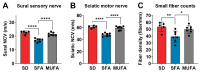
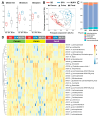
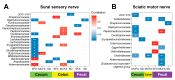
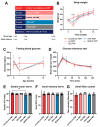
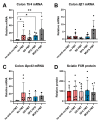
Background: Peripheral neuropathy (PN), a complication of diabetes and obesity, progresses through a complex pathophysiology. Lifestyle interventions to manage systemic metabolism are recommended to prevent or slow PN, given the multifactorial risks of diabetes and obesity. A high-fat diet rich in saturated fatty acids (SFAs) induces PN, which a diet rich in monounsaturated fatty acids (MUFAs) rescues, independent of weight loss, suggesting factors beyond systemic metabolism impact nerve health. Interest has grown in gut microbiome mechanisms in PN, which is characterized by a distinct microbiota signature that correlates with sciatic nerve lipidome.
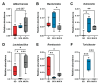
Methods: Herein, we postulated that SFA- versus MUFA-rich diet would impact gut microbiome composition and correlate with PN development. To assess causality, we performed fecal microbiota transplantation (FMT) from donor mice fed SFA- versus MUFA-rich diet to lean recipient mice and assessed metabolic and PN phenotypes.
Results: We found that the SFA-rich diet altered the microbiome community structure, which the MUFA-rich diet partially reversed. PN metrics correlated with several microbial families, some containing genera with feasible mechanisms of action for microbiome-mediated effects on PN. SFA and MUFA FMT did not impact metabolic phenotypes in recipient mice although SFA FMT marginally induced motor PN.
Conclusions: The involvement of diet-mediated changes in the microbiome on PN and gut-nerve axis may warrant further study.
Keywords: fecal microbiota transplantation; inflammation; monounsaturated fatty acid; prediabetes; saturated fatty acid.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Elafros M.A., Andersen H., Bennett D.L., Savelieff M.G., Viswanathan V., Callaghan B.C., Feldman E.L. Towards prevention of diabetic peripheral neuropathy: Clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21:922–936. doi: 10.1016/S1474-4422(22)00188-0. - DOI - PMC - PubMed
-
- O’Brien P.D., Guo K., Eid S.A., Rumora A.E., Hinder L.M., Hayes J.M., Mendelson F.E., Hur J., Feldman E.L. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis. Model. Mech. 2020;13:dmm042101. doi: 10.1242/dmm.042101. - DOI - PMC - PubMed
-
- Rumora A.E., LoGrasso G., Hayes J.M., Mendelson F.E., Tabbey M.A., Haidar J.A., Lentz S.I., Feldman E.L. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J. Neurosci. 2019;39:3770–3781. doi: 10.1523/JNEUROSCI.3173-18.2019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 1R01DK130913-00A1/NH/NIH HHS/United States
- U24 DK076169/DK/NIDDK NIH HHS/United States
- 1R00DK119366-00A1/NH/NIH HHS/United States
- U24 DK115255/DK/NIDDK NIH HHS/United States
- P30DK020572/Michigan Diabetes Research Center
- not applicable/Richard and Jane Manoogian Foundation
- not applicable/Dr. John H. Doran Neuropathy Research Initiative
- not applicable/Taubman Foundation
- P30 DK020572/DK/NIDDK NIH HHS/United States
- not applicable/Nathan and Rose Milstein Research Fund
- P30 DK063608/DK/NIDDK NIH HHS/United States
- not applicable/Sinai Medical Staff Foundation
- 1K99AG081390-00A1/NH/NIH HHS/United States
- 1R01DK076169-00A1/NH/NIH HHS/United States
- 1R01DK115255-00A1/NH/NIH HHS/United States
- K99 AG081390/AG/NIA NIH HHS/United States
- 1P30DK063608-00A20/NH/NIH HHS/United States
- R01 DK130913/DK/NIDDK NIH HHS/United States
- not applicable/NeuroNetwork for Emerging Therapies
- R00 DK119366/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

