Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals
- PMID: 40005111
- PMCID: PMC11858137
- DOI: 10.3390/molecules30040799
Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals
Abstract
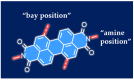
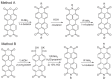
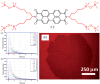
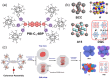
Perylene diimide (PDI), initially synthesized and explored as an organic dye, has since gained significant recognition for its outstanding optical and electronic properties. Early research primarily focused on its vibrant coloration; however, the resolution of solubility challenges has revealed its broader potential. PDIs exhibit exceptional optical characteristics, including strong absorption and high fluorescence quantum yield, along with remarkable electronic properties, such as high electron affinity and superior charge carrier mobility. Furthermore, the robust π-π stacking interactions and liquid crystalline behavior of PDIs facilitate precise their self-assembly into highly ordered structures, positioning them as valuable materials for advanced applications in optoelectronics, photonics, and nanotechnology. This article provides a comprehensive review of the progress made in the design, synthesis, and optoelectronic performance of PDI-based liquid crystals. It explores how various substituents and their placement on the PDI core impact the properties of these liquid crystal molecules and discusses the challenges and opportunities that shape this rapidly evolving class of optical materials. This review is strictly focused on PDIs and does not cover their elongated or laterally extended derivatives, nor does it include monoimide or ester compounds.
Keywords: aggregation-induced emission; charge carrier mobility; liquid crystals; perylene diimide; π-π stacking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Self-assembly, optical and electrical properties of perylene diimide dyes bearing unsymmetrical substituents at bay position.Sci Rep. 2018 May 29;8(1):8208. doi: 10.1038/s41598-018-26502-5. Sci Rep. 2018. PMID: 29844454 Free PMC article.
-
The effect of imide substituents on the optical properties of perylene diimide derivatives.Luminescence. 2018 Nov;33(7):1209-1216. doi: 10.1002/bio.3537. Epub 2018 Sep 4. Luminescence. 2018. PMID: 30178597
-
Synthesis, luminescence, and excited-state absorption properties of disubstituted perylene diimide derivatives modified at bay region.Luminescence. 2022 Feb;37(2):247-254. doi: 10.1002/bio.4166. Epub 2021 Nov 27. Luminescence. 2022. PMID: 34799958
-
Supramolecular Nanostructures Based on Perylene Diimide Bioconjugates: From Self-Assembly to Applications.Nanomaterials (Basel). 2022 Apr 5;12(7):1223. doi: 10.3390/nano12071223. Nanomaterials (Basel). 2022. PMID: 35407341 Free PMC article. Review.
-
Perylene Diimide-Based Fluorescent and Colorimetric Sensors for Environmental Detection.Sensors (Basel). 2020 Feb 9;20(3):917. doi: 10.3390/s20030917. Sensors (Basel). 2020. PMID: 32050439 Free PMC article. Review.
Cited by
-
Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals.Molecules. 2025 Jun 2;30(11):2438. doi: 10.3390/molecules30112438. Molecules. 2025. PMID: 40509325 Free PMC article.
References
-
- Liebermann C., Kardos M. Über die bei der Kernmethylierung aromatischer Basen entstehenden Acridin-Homologen. Berichte Dtsch. Chem. Ges. 1914;47:2. doi: 10.1002/cber.19140470238. - DOI
-
- Langhals H. Synthese von hochreinen Perylen-Fluoreszenzfarbstoffen in großen Mengen—Gezielte Darstellung von Atrop-Isomeren. Chem. Berichte. 1985;118:4641–4645. doi: 10.1002/cber.19851181138. - DOI
-
- Liebermann C., Kardos M. Über das Verhalten des Aceanthrenchinons gegen verdünntes Alkali. Berichte Dtsch. Chem. Ges. 1914;47:1203–1210. doi: 10.1002/cber.191404701191. - DOI
-
- Aleshinloye A.O. Multichromophoric Perylene Bisimide Dyes. Eastern Mediterranean University (EMU); Gazimağusa, Cyprus: 2009.
-
- Demmig S., Langhals H. Leichtlösliche, lichtechte Perylen-Fluoreszenzfarbstoffe. Chem. Berichte. 1988;121:225–230. doi: 10.1002/cber.19881210205. - DOI
Publication types
Grants and funding
- 92156011, 52173066/the National Natural Science Foundation of China
- 2222055/The Natural Science Foundation of Beijing
- KLLI0BJTUKF2204/The Ministry of Education Key Laboratory of Luminescence and Optical Information, Open Pro-ject
- KM202210015005/Beijing Municipal Education Commission General Scientific Research Project
- Ee202203, Ec202203, 11192024042/BIGC Project
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

