Rifampicin Repurposing Reveals Anti-Melanogenic Activity in B16F10 Melanoma Cells
- PMID: 40005210
- PMCID: PMC11858211
- DOI: 10.3390/molecules30040900
Rifampicin Repurposing Reveals Anti-Melanogenic Activity in B16F10 Melanoma Cells
Abstract
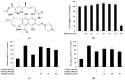
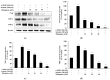
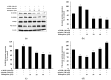
Drug repurposing is a cost-effective and innovative strategy for identifying new therapeutic applications for existing drugs, thereby shortening development timelines and accelerating the availability of treatments. Applying this approach to the development of cosmeceutical ingredients enables the creation of functional compounds with proven safety and efficacy, adding significant value to the cosmetic industry. This study evaluated the potential of rifampicin, a drug widely used for the treatment of tuberculosis and leprosy, as a cosmeceutical agent. The anti-melanogenic effects of rifampicin were assessed in B16F10 melanoma cells, showing no cytotoxicity at concentrations up to 40 µM and a significant reduction in intracellular tyrosinase activity and melanin content. Mechanistically, rifampicin reduced the expression of melanogenic enzymes, including tyrosinase, tyrosinase-related protein (TRP)-1, and TRP-2, via a protein kinase A (PKA)-dependent pathway, leading to the suppression of microphthalmia-associated transcription factor (MITF), which is a key regulator of melanogenesis. Additionally, rifampicin inhibited the p38 signaling pathway but was independent of the PI3K/protein kinase B (Akt) pathway. Furthermore, it decreased Ser9 phosphorylation, enhancing glycogen synthase kinase-3β (GSK-3β) activity, promoted β-catenin phosphorylation, and facilitated β-catenin degradation, collectively contributing to the inhibition of melanin synthesis. To evaluate the topical applicability of rifampicin, primary human skin irritation tests were conducted, and no adverse effects were observed at concentrations of 20 µM and 40 µM. These findings demonstrate that rifampicin inhibits melanogenesis through multiple signaling pathways, including PKA, MAPKs, and GSK-3β/β-catenin. This study highlights the potential of rifampicin to be repurposed as a topical agent for managing hyperpigmentation disorders, offering valuable insights into novel therapeutic strategies for pigmentation-related conditions.
Keywords: B16F10; PI3K/Akt pathway; PKA pathway; cosmeceutical; drug repurposing; hyperpigmentation; melanogenesis; rifampicin; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Jonker A.H., O’Connor D., Cavaller-Bellaubi M., Fetro C., Gogou M., ’t Hoen P.A.C., de Kort M., Stone H., Valentine N., Pasmooij A.M.G. Drug repurposing for rare: Progress and opportunities for the rare disease community. Front. Med. 2024;11:1352803. doi: 10.3389/fmed.2024.1352803. - DOI - PMC - PubMed
-
- Pinzi L., Bisi N., Rastelli G. How drug repurposing can advance drug discovery: Challenges and opportunities. Front. Drug Discov. 2024;4:1460100. doi: 10.3389/fddsv.2024.1460100. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

