Computational Analysis of the Kinetic Requirements for Coupled Reaction Systems
- PMID: 40005221
- PMCID: PMC11858731
- DOI: 10.3390/molecules30040911
Computational Analysis of the Kinetic Requirements for Coupled Reaction Systems
Abstract
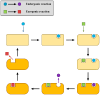
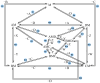
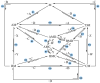
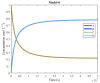
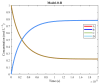
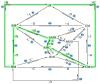
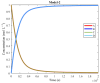
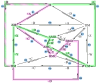
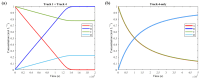
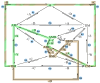
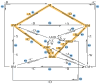
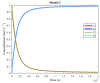
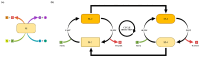
The art of designing coupling systems to drive reactions for endergonic synthesis is a subject of great interest in the scientific community, but it still presents major challenges. The aim of this kinetic study was to run simulations in COPASI 4.39 to test the behavior of hypothetical models for a system that couples two independent reactions, one exergonic and the other endergonic. In our computational study, we unraveled the qualitative and quantitative conditions that allow and benefit coupling, considering all possible reaction pathways within the network. Optimal conditions were reached by assigning favorable directionalities and low activation energies to six reaction steps within a network that featured twenty reaction steps. Moreover, different models were designed and tested in order to investigate the availability of coupling with different reaction steps.
Keywords: computational model; endergonic synthesis; kinetics; simulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Deffner S., Bonança M.V.S. Thermodynamic control—An old paradigm with new applications. EPL. 2020;131:20001. doi: 10.1209/0295-5075/131/20001. - DOI
-
- Marsden S.R., Mestrom L., McMillan D.G.G., Hanefeld U. Thermodynamically and Kinetically Controlled Reactions in Biocatalysis—From Concepts to Perspectives. ChemCatChem. 2020;12:426–437. doi: 10.1002/cctc.201901589. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

