PK/PD Analysis of High-Dose Daptomycin Use in the Treatment of Bone and Joint Infections: Data from a Real-World Setting
- PMID: 40005671
- PMCID: PMC11858051
- DOI: 10.3390/microorganisms13020304
PK/PD Analysis of High-Dose Daptomycin Use in the Treatment of Bone and Joint Infections: Data from a Real-World Setting
Abstract
Background: Daptomycin is widely used in bone and joint infections (BJIs) caused by Gram-positive cocci. The pharmacokinetics of daptomycin are characterized by relevant variability in terms of drug exposure. Due to these pharmacological properties, the dosing suggested by the Summary of medical Product Characteristics could result in sub-therapeutic or toxic concentrations, especially considering the high doses recommended for BJIs. Therapeutic Drug Monitoring (TDM) of daptomycin helps clinicians in verifying the patient's exposure, due to the lack of pharmacokinetic/pharmacodynamic (PK/PD) data in this clinical setting.
Methods: We retrospectively analyzed 170 daptomycin plasma concentrations of 77 patients with BJIs from July 2022 to December 2023. We focused on the pharmacokinetics of daptomycin to investigate when drug plasma concentrations achieved adequate PK/PD targets.
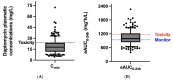
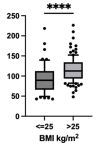
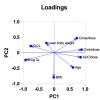
Results: In the first TDM, 7.8% of patients were underexposed according to the estimated area under the curve (eAUC0-24h < 666 mg·h/L), whereas 35.1% were on target according to both the eAUC and trough plasma concentration (eAUC0-24h 666 - 939 mg·h/L; Cmin < 24.3 mg/L). The patients who were overexposed had trough plasma concentrations > 24.3 mg/L (27.3%) or eAUC0-24h > 1174 mg·h/L (33.8%). Differences in drug exposure were observed according to weight and sex.
Conclusions: Due to the difficult management of this drug's dosing, analyzing daptomycin plasma concentrations through TDM represents a powerful tool in BJIs.
Keywords: bone and joint infections; daptomycin; infectious diseases; pharmacodynamic; pharmacokinetics; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflicts of interest related to the topics discussed in this manuscript.
Figures



References
-
- Osmon D.R., Berbari E.F., Berendt A.R., Lew D., Zimmerli W., Steckelberg J.M., Rao N., Hanssen A., Wilson W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. - DOI - PubMed
-
- Karpiński R., Szabelski J., Krakowski P., Jonak J., Falkowicz K., Jojczuk M., Nogalski A., Przekora A. Effect of Various Admixtures on Selected Mechanical Properties of Medium Viscosity Bone Cements: Part 2—Hydroxyapatite. Compos. Struct. 2024;343:118308. doi: 10.1016/j.compstruct.2024.118308. - DOI
LinkOut - more resources
Full Text Sources

