Role of msbB Gene in Physiology and Pathogenicity of Vibrio parahaemolyticus
- PMID: 40005752
- PMCID: PMC11857884
- DOI: 10.3390/microorganisms13020386
Role of msbB Gene in Physiology and Pathogenicity of Vibrio parahaemolyticus
Abstract
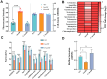
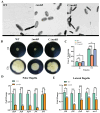
The msbB gene, encoding a lipid A phosphatease, is crucial for lipopolysaccharide (LPS) synthesis in Gram-negative bacteria and plays a critical role in their virulence. This study investigated the role of msbB in Vibrio parahaemolyticus, a significant marine pathogen causing gastroenteritis in humans and infections in aquatic animals. We constructed an msbB deletion mutant (ΔmsbB) and a complementary strain (CΔmsbB) using homologous recombination. The growth, outer membrane permeability, stress and antibiotic sensitivity, biofilm formation, swarming motility, and virulence of the wild-type (WT), ΔmsbB, and CΔmsbB strains were assessed. Additionally, the pathogenicity of ΔmsbB was evaluated using L. vannamei shrimp models. The results showed that the msbB gene was successfully deleted and complemented, and its deletion did not impair bacterial growth. However, the ΔmsbB strain exhibited an increased outer membrane permeability, reduced resistance to stresses and antibiotics, defective biofilm formation, and a reduced swarming motility. In a Tetrahymena co-culture, the ΔmsbB strain showed attenuated virulence. In shrimp infected with the ΔmsbB strain, the cumulative mortality rate was 22%, significantly lower than the 62% observed in the WT strain. Moreover, the expression levels of immune-related genes in the shrimp hepatopancreas were significantly lower in the ΔmsbB group, indicating a significant reduction in infection capability and pathogenicity. These findings indicate that the msbB gene is critical for the virulence of V. parahaemolyticus and suggest that msbB is a potential target for therapeutic interventions and vaccine development against V. parahaemolyticus infections.
Keywords: V. parahaemolyticus; msbB gene; pathogenicity; shrimp infection; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Deletion of the Braun lipoprotein-encoding gene and altering the function of lipopolysaccharide attenuate the plague bacterium.Infect Immun. 2013 Mar;81(3):815-28. doi: 10.1128/IAI.01067-12. Epub 2012 Dec 28. Infect Immun. 2013. PMID: 23275092 Free PMC article.
-
Construction and characterization of different hemolysin gene deletion strains in Vibrio parahaemolyticus (ΔhlyA, ΔhlyIII) and evaluation of their virulence.J Invertebr Pathol. 2024 Nov;207:108210. doi: 10.1016/j.jip.2024.108210. Epub 2024 Sep 27. J Invertebr Pathol. 2024. PMID: 39343130
-
Salmonella enterica serovar Typhimurium ΔmsbB triggers exacerbated inflammation in Nod2 deficient mice.PLoS One. 2014 Nov 25;9(11):e113645. doi: 10.1371/journal.pone.0113645. eCollection 2014. PLoS One. 2014. PMID: 25423082 Free PMC article.
-
Optimized swarming motility assay to identify anti-virulence products against Vibrio parahaemolyticus, a pathogen of farmed shrimp.MethodsX. 2024 Feb 20;12:102622. doi: 10.1016/j.mex.2024.102622. eCollection 2024 Jun. MethodsX. 2024. PMID: 38425495 Free PMC article.
-
BolA-like protein (IbaG) promotes biofilm formation and pathogenicity of Vibrio parahaemolyticus.Front Microbiol. 2024 Jul 31;15:1436770. doi: 10.3389/fmicb.2024.1436770. eCollection 2024. Front Microbiol. 2024. PMID: 39144210 Free PMC article.
Cited by
-
A critical role for Vibrio parahaemolyticus LPS to mediate evasion of host immune response during infection.Proc Natl Acad Sci U S A. 2025 Aug 19;122(33):e2426547122. doi: 10.1073/pnas.2426547122. Epub 2025 Aug 13. Proc Natl Acad Sci U S A. 2025. PMID: 40802686 Free PMC article.
References
-
- Bai Y., Yang Q., Sun Y., Li F., Sun J., Yang S., Yang D., Peng Z., Yang B., Xu J., et al. Antimicrobial susceptibility and genomic characterization of Vibrio parahaemolyticus isolated from aquatic foods in 15 provinces, China, 2020. Int. J. Food Microbiol. 2024;418:110737. doi: 10.1016/j.ijfoodmicro.2024.110737. - DOI - PubMed
-
- Rezny B.R., Evans D.S. StatPearls. StatPearls Publishing LLC.; St. Petersburg, FL, USA: 2025. Vibrio parahaemolyticus Infection. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

