Improved Natamycin Production in Streptomyces gilvosporeus Through Mutagenesis and Enhanced Nitrogen Metabolism
- PMID: 40005756
- PMCID: PMC11857858
- DOI: 10.3390/microorganisms13020390
Improved Natamycin Production in Streptomyces gilvosporeus Through Mutagenesis and Enhanced Nitrogen Metabolism
Abstract
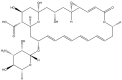
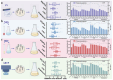
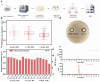
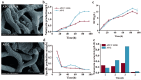
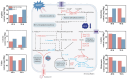
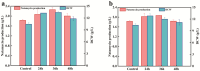
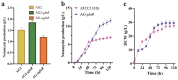
Natamycin is a polyene macrocyclic antibiotic extensively used in food, medical, and agricultural industries. However, its high production cost and low synthetic efficiency fail to meet the growing market demand. Therefore, enhancing the production of natamycin-producing strains is crucial for achieving its industrial-scale production. This study systematically evaluated 16 mutagenesis methods and identified atmospheric and room temperature plasma mutagenesis combined with 2-deoxyglucose tolerance screening as the optimal strategy for enhancing natamycin production. A high-yield mutant strain, AG-2, was obtained, achieving an 80% increase in natamycin production (1.53 g/L) compared to the original strain. Metabolic analysis revealed that glycolysis and the pentose phosphate pathway were enhanced in AG-2, while the tricarboxylic acid cycle was weakened, significantly increasing the supply of precursors such as acetyl-CoA, methylmalonyl-CoA, and the reducing power of NADPH. Additionally, overexpression of the nitrogen metabolism regulatory gene glnR promoted the supply of glutamate and glutamine, further increasing natamycin production in AG-2 to 1.85 g/L. In a 5 L fermenter, the engineered strain AG-glnR achieved a final natamycin production of 11.50 g/L, 1.67 times higher than the original strain. This study is the first to combine mutagenesis with nitrogen metabolism regulation, effectively enhancing natamycin production and providing a novel approach for the efficient synthesis of other polyene antibiotics.
Keywords: 2-deoxyglucose; ARTP mutagenesis; Streptomyces gilvosporeus; natamycin; nitrogen metabolism regulation.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Enhanced Natamycin production in Streptomyces gilvosporeus through phosphate tolerance screening and transcriptome-based analysis of high-yielding mechanisms.Microb Cell Fact. 2025 Apr 2;24(1):79. doi: 10.1186/s12934-025-02696-y. Microb Cell Fact. 2025. PMID: 40176084 Free PMC article.
-
[Engineering the precursor supply pathway in Streptomyces gilvosporeus for overproduction of natamycin].Sheng Wu Gong Cheng Xue Bao. 2022 Dec 25;38(12):4630-4643. doi: 10.13345/j.cjb.220351. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 36593198 Chinese.
-
Enhancement of natamycin production by combining ARTP mutagenesis with temperature control strategy development in Streptomyces gilvosporeus.Bioprocess Biosyst Eng. 2025 May;48(5):817-827. doi: 10.1007/s00449-025-03145-1. Epub 2025 Mar 18. Bioprocess Biosyst Eng. 2025. PMID: 40100347
-
[Advances in the biosynthesis of natamycin and its regulatory mechanisms].Sheng Wu Gong Cheng Xue Bao. 2021 Apr 25;37(4):1107-1119. doi: 10.13345/j.cjb.200394. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 33973428 Review. Chinese.
-
Biotechnological production and application of the antibiotic pimaricin: biosynthesis and its regulation.Appl Microbiol Biotechnol. 2016 Jan;100(1):61-78. doi: 10.1007/s00253-015-7077-0. Epub 2015 Oct 29. Appl Microbiol Biotechnol. 2016. PMID: 26512010 Free PMC article. Review.
References
-
- Prajna N.V., Mascarenhas J., Krishnan T., Reddy P.R., Prajna L., Srinivasan M., Vaitilingam C.M., Hong K.C., Lee S.M., McLeod S.D., et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch. Ophthalmol. 2010;128:672–678. doi: 10.1001/archophthalmol.2010.102. - DOI - PMC - PubMed
-
- Civelek I., Cagri-Mehmetoglu A. Determination of antifungal effect of edible coatings containing Williopsis saturnus var saturnus against yeast and mold growth on kashar cheese. J. Food Sci. 2019;84:311–318. - PubMed
-
- El-Nabarawi M.A., Abd El Rehem R.T., Teaima M., Abary M., El-Mofty H.M., Khafagy M.M., Lotfy N.M., Salah M. Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev. Ind. Pharm. 2019;45:922–936. doi: 10.1080/03639045.2019.1579827. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

