Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies
- PMID: 40005812
- PMCID: PMC11858063
- DOI: 10.3390/microorganisms13020447
Mycobacterium abscessus subsp. massiliense: Biofilm Formation, Host Immune Response, and Therapeutic Strategies
Abstract
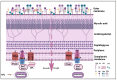
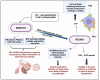
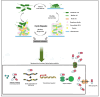
Infection by Mycobacterium abscessus subsp. massiliense poses a growing public health threat, especially to immunocompromised individuals. The pathogenicity of this mycobacterium is directly linked to its ability to form biofilms, complex structures that confer resistance to antibiotics and the host immune response. The extracellular matrix of the biofilm acts as a physical barrier, hindering the penetration of drugs and the action of the immune system, while also inducing a slow-growth state that reduces susceptibility to antibiotics. Current therapies, which involve prolonged use of multiple antibiotics, are often ineffective and cause significant side effects. Therefore, it is essential to explore new strategies targeting bacterial resistance and biofilm destruction. This narrative review explores the biofilm-forming capacity of Mycobacterium abscessus subsp. massiliense and the potential of novel therapeutic strategies. Promising approaches include inhibiting biofilm formation, developing drugs with improved penetration of the extracellular matrix, combination therapies with agents that destabilize the biofilm structure, and modulating the host immune response. Investing in research and development of new therapeutic strategies is essential to combat this resistant bacterium and improve patient outcomes.
Keywords: Mycobacterium abscessus subsp. massiliense; biofilm; immune response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kolpen M., Jensen P.Ø., Qvist T., Kragh K.N., Ravnholt C., Fritz B.G., Johansen U.R., Bjarnsholt T., Hoiby N. Biofilms of Mycobacterium abscessus Complex Can Be Sensitized to Antibiotics by Disaggregation and Oxygenation. Antimicrob. Agents Chemother. 2019;64:e01212-19. doi: 10.1128/AAC.01212-19. - DOI - PMC - PubMed
-
- De K., Belardinelli J.M., Pandurangan A.P., Ehianeta T., Lian E., Palčeková Z., Lam H., Gonzalez-Juarrero M., Bryant J.M., Blundell T.L., et al. Lipoarabinomannan modification as a source of phenotypic heterogeneity in host-adapted Mycobacterium abscessus isolates. Proc. Natl. Acad. Sci. USA. 2024;121:e2403206121. doi: 10.1073/pnas.2403206121. - DOI - PMC - PubMed
-
- Mauch R.M., Jensen P.Ø., Qvist T., Kolpen M., Moser C., Pressler T., da Silva M.T.N., Høiby N. Adaptive Immune Response to Mycobacterium abscessus Complex (MABSC) in Cystic Fibrosis and the Implications of Cross-Reactivity. Front. Cell. Infect. Microbiol. 2022;12:858398. doi: 10.3389/fcimb.2022.858398. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

