Enhancing Tetrahydrocannabinol's Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011
- PMID: 40005963
- PMCID: PMC11858241
- DOI: 10.3390/ph18020148
Enhancing Tetrahydrocannabinol's Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011
Abstract
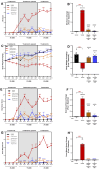
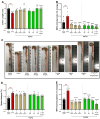
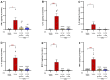
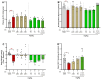
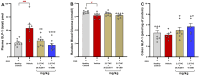
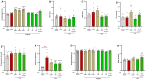
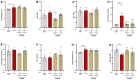
Background/Objectives: Current inflammatory bowel disease (IBD) treatments focus on symptomatic relief, highlighting the need for innovative approaches. Dysregulation of the cannabinoid 1 (CB1) receptor, part of the endocannabinoid system, is linked to colitis. While tetrahydrocannabinol (THC) alleviates colitis via CB1 activation, its psychotropic effects limit clinical use. ZCZ011, a CB1R allosteric modulator, and cannabidiol (CBD), a non-psychoactive cannabinoid, offer alternatives. This study investigated combining sub-therapeutic THC doses with ZCZ011 or CBD in a murine model of dextran sodium sulphate (DSS)-induced colitis. Methods: Acute colitis was induced with 4% DSS for 7 days, followed by 3 days of water. Chronic colitis was modelled over 24 days with alternating DSS concentrations. The combination of 2.5 mg/kg THC with 20 mg/kg ZCZ011 or 10 mg/kg CBD was evaluated. Key markers were assessed to determine efficacy and safety, including disease activity index (DAI), inflammation, cytokine levels, GLP-1, and organ health. Results: DSS-induced colitis resulted in increased DAI scores, cytokines, organ inflammation and dysregulation of GLP-1 and ammonia. THC at 10 mg/kg significantly improved colitis markers but was ineffective at 2.5 and 5 mg/kg. ZCZ011 alone showed transient effects. However, combining 2.5 mg/kg THC with either 20 mg/kg ZCZ011 or 10 mg/kg CBD significantly alleviated colitis markers, restored colon integrity and reestablished GLP-1 homeostasis. This combination also maintained favourable haematological and biochemical profiles, including a notable reduction in colitis-induced elevated ammonia levels. Conclusions: This study demonstrates the synergistic potential of low-dose THC combined with CBD or ZCZ011 as a novel, effective and safer therapeutic strategy for ulcerative colitis.
Keywords: CB1R allosteric modulator; DSS-induced ulcerative colitis; ZCZ011; ammonia; cannabidiol (CBD); cannabinoid 1 receptor (CB1R); glucagon-like peptide 1 (GLP-1); inflammatory bowel disease; tetrahydrocannabinol (THC).
Conflict of interest statement
Curtin University received financial support from Little Green Pharma Ltd. to fund M.F. in this study. M.F. is a member of LIPOVEXA S.r.l., a spin-off company focused on developing innovative treatments for diabetes, obesity and liver health. L.N.W. was employed by Little Green Pharma Pty Ltd. at the time of the research. D.T. received PhD stipends from Little Green Pharma Ltd. No additional relationships or activities are reported that could have influenced the findings of this work.
Figures


Similar articles
-
Comprehensive Assessment of Cannabidiol and HU308 in Acute and Chronic Colitis Models: Efficacy, Safety, and Mechanistic Innovations.Cells. 2024 Dec 5;13(23):2013. doi: 10.3390/cells13232013. Cells. 2024. PMID: 39682761 Free PMC article.
-
CB1 positive allosteric modulation attenuates Δ9-THC withdrawal and NSAID-induced gastric inflammation.Pharmacol Biochem Behav. 2019 Feb;177:27-33. doi: 10.1016/j.pbb.2018.12.009. Epub 2018 Dec 28. Pharmacol Biochem Behav. 2019. PMID: 30597181 Free PMC article.
-
The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis.Br J Pharmacol. 2010 Jun;160(3):712-23. doi: 10.1111/j.1476-5381.2010.00791.x. Br J Pharmacol. 2010. PMID: 20590574 Free PMC article.
-
The Modulatory Effects and Therapeutic Potential of Cannabidiol in the Gut.Cells. 2024 Sep 26;13(19):1618. doi: 10.3390/cells13191618. Cells. 2024. PMID: 39404382 Free PMC article. Review.
-
Delta-9-tetrahydrocannabinol/cannabidiol (Sativex®): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis.Drugs. 2014 Apr;74(5):563-78. doi: 10.1007/s40265-014-0197-5. Drugs. 2014. PMID: 24671907 Review.
Cited by
-
Cannabis: Zone Aspects of Raw Plant Components in Sport-A Narrative Review.Nutrients. 2025 Feb 28;17(5):861. doi: 10.3390/nu17050861. Nutrients. 2025. PMID: 40077729 Free PMC article. Review.
-
Targeting the Endocannabinoidome: A Novel Approach to Managing Extraintestinal Complications in Inflammatory Bowel Disease.Pharmaceuticals (Basel). 2025 Mar 27;18(4):478. doi: 10.3390/ph18040478. Pharmaceuticals (Basel). 2025. PMID: 40283915 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

