Drug Development in Inflammatory Bowel Diseases: What Is Next?
- PMID: 40006003
- PMCID: PMC11858795
- DOI: 10.3390/ph18020190
Drug Development in Inflammatory Bowel Diseases: What Is Next?
Abstract
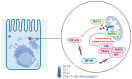
Background/Objectives: Inflammatory bowel diseases (IBDs), which include Crohn's disease (CD) and ulcerative colitis (UC), are chronic conditions requiring long-term therapy to maintain remission and improve quality of life. Despite the approval of numerous drugs, IBD continues to present treatment challenges. This review aims to summarize novel therapeutic target agents in phases II and III of development, including sphingosine-1-phosphate receptor modulators (S1P), anti-interleukin-23 (IL-23), and other small molecules and monoclonal antibodies currently under investigation (e.g., anti-TL1A, obefazimod, NX-13, RIPK-inhibitors). Methods: A comprehensive literature search was conducted up to December 2024 to identify relevant articles published in English over the past three-five years, focusing on phase II/III studies for UC and CD. The search included databases such as PubMed, Google Scholar, and the ClinicalTrials.gov portal. Results: Clinical trials underline the potential of novel immunomodulators, including anti-TL1A, obefazimod, NX-13, RIPK inhibitors, and anti-IL-23p19 agents, as promising therapeutic options for IBD. Anti-IL23p19 therapies, such as risankizumab and mirikizumab, alongside guselkumab, exemplify this class's growing clinical relevance. While some are already in clinical use, others are nearing approval. Conclusions: Ongoing research into long-term safety and the development of personalized treatment strategies remains pivotal to enhance outcomes. Patient stratification and the strategic positioning of these therapies within the expanding treatment landscape are critical for optimizing their clinical impact.
Keywords: Crohn’s disease; NX-13; S1P modulators; anti IL-23; anti-TL1A antibodies; obefazimod; ulcerative colitis.
Conflict of interest statement
A. Armuzzi has received consulting fees from AbbVie, Allergan, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz and Takeda; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, and Tigenix; and research support from Biogen, MSD, Takeda, and Pfizer. C Bezzio received lecture fees and served as a consultant for Takeda, MSD, Ferring, Abbvie, Galapagos, Celltrion, Janssen, and Eli-Lilly. R. Gabbiadini has received speaker’s fees from Pfizer, MSD, Eli-Lilly, Janssen, and Celltrion. A. Dal Buono has received speaker’s fees from AbbVie, Galapagos, Eli-Lilly, Janssen, and Celltrion, and consulting fees from Ferring. L. Loy has received speaker’s fees from AbbVie, Galapagos, Eli-Lilly, Janssen, and Celltrion. G Privitera has received speaker’s fee from Janssen and Alphasigma. L. Petronio, G Migliorisi, and M Ferraris declare no conflict of interest.
Similar articles
-
Immunity in digestive diseases: new drugs for inflammatory bowel disease treatment-insights from Phase II and III trials.J Gastroenterol. 2024 Sep;59(9):761-787. doi: 10.1007/s00535-024-02130-x. Epub 2024 Jul 9. J Gastroenterol. 2024. PMID: 38980426 Free PMC article. Review.
-
Advancing therapeutic frontiers: a pipeline of novel drugs for luminal and perianal Crohn's disease management.Therap Adv Gastroenterol. 2024 Dec 19;17:17562848241303651. doi: 10.1177/17562848241303651. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39711916 Free PMC article. Review.
-
Review article: emerging drug therapies in inflammatory bowel disease.Aliment Pharmacol Ther. 2022 Apr;55(7):789-804. doi: 10.1111/apt.16785. Epub 2022 Feb 15. Aliment Pharmacol Ther. 2022. PMID: 35166398 Review.
-
IL23p19 therapies for moderately-to-severely active ulcerative colitis.Expert Opin Biol Ther. 2025 Apr;25(4):363-378. doi: 10.1080/14712598.2025.2480258. Epub 2025 Mar 24. Expert Opin Biol Ther. 2025. PMID: 40082083 Review.
-
Promising phase II biologics for future Crohn's disease therapy.Expert Opin Investig Drugs. 2023 Jan-Jun;32(6):495-507. doi: 10.1080/13543784.2023.2219386. Epub 2023 May 31. Expert Opin Investig Drugs. 2023. PMID: 37249522 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous