Protective Effects of Oleanolic Acid on Human Keratinocytes: A Defense Against Exogenous Damage
- PMID: 40006051
- PMCID: PMC11859478
- DOI: 10.3390/ph18020238
Protective Effects of Oleanolic Acid on Human Keratinocytes: A Defense Against Exogenous Damage
Abstract
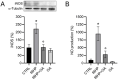
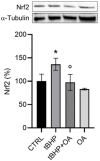
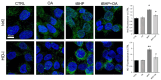
Background/objectives: Aging leads to increased oxidative stress and chronic inflammation in the skin, which contribute to various disorders such as dermatitis and cancer. This study explores the cytoprotective effects of oleanolic acid (OA), a natural triterpenoid compound known for its potential in mitigating oxidative damage, on human keratinocyte (HaCaT) cells exposed to oxidative stress from tert-butyl hydroperoxide (tBHP). Methods: Using in vitro experiments, we assessed cell viability, reactive oxygen species (ROS) levels, nitric oxide (NO) production, and protein expression following OA pre-treatment. Advanced imaging techniques were employed to visualize protein localization. Results: Results demonstrated that OA significantly improved cell viability and reduced intracellular ROS levels compared with those in controls. Additionally, OA inhibited inducible nitric oxide synthase (iNOS) expression and subsequent nitric oxide release, indicating a modulation of inflammatory responses. Notably, while tBHP activated the Nrf2/HO-1 signaling pathway, OA did not enhance this response, suggesting that OA exerts cytoprotective effects through mechanisms independent of Nrf2 activation. Conclusion: OA shows promise in protecting HaCaT cells from tBHP-induced oxidative stress, highlighting its potential role in promoting skin health and addressing aging-related damage. The study proposes that OA operates through pathways distinct from Nrf2 and MAPKs, paving the way for new therapeutic strategies aimed at improving skin health against oxidative stress.
Keywords: HaCaT cells; oleanolic acid; oxidative stress; tert-butyl-hydroperoxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Liu H.-M., Cheng M.-Y., Xun M.-H., Zhao Z.-W., Zhang Y., Tang W., Cheng J., Ni J., Wang W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023;24:3755. doi: 10.3390/ijms24043755. - DOI - PMC - PubMed
-
- Aranda-Rivera A.K., Cruz-Gregorio A., Arancibia-Hernández Y.L., Hernández-Cruz E.Y., Pedraza-Chaverri J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen. 2022;2:437–478. doi: 10.3390/oxygen2040030. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

