The Therapeutic Potential of Spirooxindoles in Cancer: A Focus on p53-MDM2 Modulation
- PMID: 40006086
- PMCID: PMC11859340
- DOI: 10.3390/ph18020274
The Therapeutic Potential of Spirooxindoles in Cancer: A Focus on p53-MDM2 Modulation
Abstract
The p53, often referred to as the "guardian of the genome", is a well-established tumor-suppressor protein that plays a critical role in regulating the cell cycle, DNA repair, differentiation, and apoptosis, with its activity primarily modulated by the MDM2 protein (murine double minute 2, also known as HDM2 in humans). Disrupting the protein-protein interaction between p53 and MDM2 represents a promising therapeutic strategy for developing anticancer agents. Recent studies have shown that several spirooxindole-containing compounds exhibit significant antitumor properties, primarily by inhibiting the p53-MDM2 interaction. This review provides an overview of structure-based spirooxindoles that could have therapeutic potential. It highlights findings from the past decade concerning their antiproliferative properties and implications for interfering with the p53-MDM2 interaction. The discussion includes various analogs of spirooxindoles as promising candidates for optimizing leads in drug discovery programs aimed at developing novel and clinically effective agents.
Keywords: MDM2; cancer; p53; spirooxindole.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




























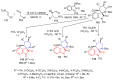


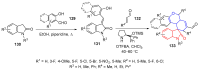



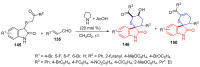
References
-
- Koren E., Fuchs Y. Modes of regulated cell death in cancer. Cancer Discov. 2021;11:245–265. doi: 10.1158/2159-8290.CD-20-0789. - DOI - PubMed
-
- Gollner A., Rudolph D., Arnhof H., Bauer M., Blake S.M., Boehmelt G., Cockroft X.-L., Dahmann G., Ettmayer P., Gerstberger T., et al. Discovery of novel spiro[3H-indole-3,2′-pyrrolidin]-2(1H)-one compounds as chemically stable and orally active inhibitors of the MDM2-p53 interaction. J. Med. Chem. 2016;59:10147–10162. doi: 10.1021/acs.jmedchem.6b00900. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

