Iodinated Copper-Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy
- PMID: 40006516
- PMCID: PMC11858929
- DOI: 10.3390/pharmaceutics17020149
Iodinated Copper-Cysteamine Nanoparticles as Radiosensitizers for Tumor Radiotherapy
Abstract
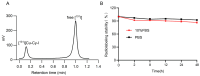
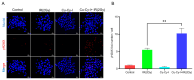
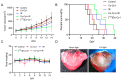
Background/Objectives: Radiotherapy is a widely applied first-line clinical treatment modality of cancer. Copper-cysteamine (Cu-Cy) nanoparticles represent a new type of photosensitizer that demonstrates significant anti-tumor potential by X-ray-induced photodynamic therapy. Iodide is a high-Z element with superior X-ray absorption ability and has the β-decay radiotherapeutic nuclide, 131I, which emits Cherenkov light. In this study we aimed to investigate the X-ray-induced photodynamic therapy potential of iodinated Cu-Cy (Cu-Cy-I) nanoparticles and also explore the local treatment efficacy of 131I-labeled Cu-Cy-I ([131I]Cu-Cy-I) nanoparticles. Methods: The synthesis of [131I]Cu-Cy-I nanoparticles was performed with [131I]I- anions. The in vitro radiobiological effects on tumor cells incubated with Cu-Cy-I nanoparticles by X-ray irradiation were investigated. The in vivo tumor growth-inhibitory effects of the combination of Cu-Cy-I nanoparticles with X-ray radiotherapy and [131I]Cu-Cy-I nanoparticles were evaluated with 4T1 tumor-xenografted mice. Results: The in vitro experiment results indicated that the X-ray irradiation with the presence of Cu-Cy-I nanoparticles produced a higher intracellular reactive oxygen species (ROS) level and more DNA damage of 4T1 cells and showed a stronger tumor cell killing ability compared to X-ray irradiation alone. The in vivo experimental results with 4T1 breast carcinoma-bearing mice showed that the combination of an intratumoral injection of Cu-Cy-I nanoparticles and X-ray radiotherapy enhanced the tumor growth-inhibitory effect and prolonged the mice's lives. Conclusions: Cu-Cy-I nanoparticles have good potential as new radiosensitizers to enhance the efficacy of external X-ray radiotherapy. However, the efficacy of local treatment with [131I]Cu-Cy-I nanoparticles at a low 131I dose was not verified. The effective synthesis of smaller sizes of nanoparticles is necessary for further investigation of the radiotherapy potential of [131I]Cu-Cy-I nanoparticles.
Keywords: 131I; X-ray-induced photodynamic therapy; copper–cysteamine nanoparticles; radiosensitization; radiotherapy.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Investigation of Copper Cysteamine Nanoparticles as a New Type of Radiosensitiers for Colorectal Carcinoma Treatment.Sci Rep. 2017 Aug 24;7(1):9290. doi: 10.1038/s41598-017-09375-y. Sci Rep. 2017. PMID: 28839163 Free PMC article.
-
A facile method for the synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment.J Mater Chem B. 2019 Nov 14;7(42):6630-6642. doi: 10.1039/c9tb01566c. Epub 2019 Oct 8. J Mater Chem B. 2019. PMID: 31591609
-
Study of copper-cysteamine based X-ray induced photodynamic therapy and its effects on cancer cell proliferation and migration in a clinical mimic setting.Bioact Mater. 2021 May 30;7:504-514. doi: 10.1016/j.bioactmat.2021.05.016. eCollection 2022 Jan. Bioact Mater. 2021. PMID: 34466749 Free PMC article.
-
Investigation of copper-cysteamine nanoparticles as a new photosensitizer for anti-hepatocellular carcinoma.Cancer Biol Ther. 2019;20(6):812-825. doi: 10.1080/15384047.2018.1564568. Epub 2019 Feb 6. Cancer Biol Ther. 2019. PMID: 30727796 Free PMC article.
-
Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications.Int J Mol Sci. 2019 Dec 31;21(1):273. doi: 10.3390/ijms21010273. Int J Mol Sci. 2019. PMID: 31906108 Free PMC article. Review.
References
-
- Zhu H., Chua M.L.K., Chitapanarux I., Kaidar-Person O., Mwaba C., Alghamdi M., Rodríguez Mignola A., Amrogowicz N., Yazici G., Bourhaleb Z., et al. Global radiotherapy demands and corresponding radiotherapy-professional workforce requirements in 2022 and predicted to 2050: A population-based study. Lancet Glob. Health. 2024;12:e1945–e1953. doi: 10.1016/S2214-109X(24)00355-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

