Exploratory Study on Nanoparticle Co-Delivery of Temozolomide and Ligustilide for Enhanced Brain Tumor Therapy
- PMID: 40006558
- PMCID: PMC11858958
- DOI: 10.3390/pharmaceutics17020191
Exploratory Study on Nanoparticle Co-Delivery of Temozolomide and Ligustilide for Enhanced Brain Tumor Therapy
Abstract
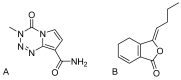
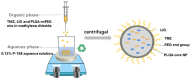
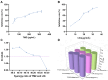
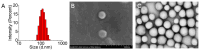
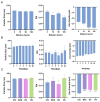
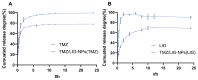
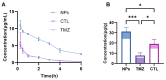
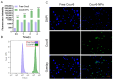
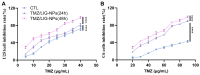
Background: Temozolomide (TMZ) is the first-line therapy for glioblastoma (GBM), but its clinical efficacy is limited by its short half-life, poor brain targeting, adverse side effects, and the development of drug resistance. Ligustilide (LIG) has been shown to enhance blood-brain barrier permeability and reduce P-glycoprotein activity, thereby potentiating the synergistic effect of TMZ against GBM. Methods: The dual-drug-loaded nanoparticles encapsulating both TMZ and LIG (TMZ/LIG-NPs) were prepared using Poly (d,l-lactic-co-glycolide)-monomethoxy poly (ethylene glycol) (PLGA-mPEG). The physicochemical properties of the NPs, including particle size and zeta potential, were characterized. Cellular uptake of NPs was evaluated using flow cytometry and fluorescence staining. The pharmacokinetic profile and cytotoxicity of TMZ/LIG-NPs were compared to those of free TMZ and a mixture of TMZ and LIG in rat and glioma cells, respectively. Results: The mean particle size of TMZ/LIG-NPs was 117.6 ± 0.7 nm, with a zeta potential of -26.5 ± 0.4 mV. Cellular uptake of NPs was significantly higher than that of free drug in U251 cells. Encapsulation of TMZ in NPs significantly increased its half-life by 1.62-fold compared to free TMZ and significantly improved its pharmacokinetic profile. Moreover, the storage stability of the TMZ/LIG-NPs solution was extended to one month. The toxicity of TMZ/LIG-NPs to glioma cells C6 and U251 was markedly enhanced compared to the mixture of TMZ and LIG. Conclusions: The development of TMZ/LIG-NPs using PLGA-mPEG effectively enhanced the stability and efficacy of both TMZ and LIG. This dual drug-loaded nanoparticle system represents a promising strategy for glioblastoma therapy.
Keywords: dual drug-loaded nanoparticles; glioblastoma multiforme; ligustilide; temozolomide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Temozolomide hexadecyl ester targeted plga nanoparticles for drug-resistant glioblastoma therapy via intranasal administration.Front Pharmacol. 2022 Aug 17;13:965789. doi: 10.3389/fphar.2022.965789. eCollection 2022. Front Pharmacol. 2022. PMID: 36059989 Free PMC article.
-
Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(lactic-co-glycolic acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells.Mol Pharm. 2020 Nov 2;17(11):4386-4400. doi: 10.1021/acs.molpharmaceut.0c00856. Epub 2020 Oct 20. Mol Pharm. 2020. PMID: 33079558
-
Oncolytic Newcastle Disease Virus Co-Delivered with Modified PLGA Nanoparticles Encapsulating Temozolomide against Glioblastoma Cells: Developing an Effective Treatment Strategy.Molecules. 2022 Sep 6;27(18):5757. doi: 10.3390/molecules27185757. Molecules. 2022. PMID: 36144488 Free PMC article.
-
Self-Assembled systems for Nose-to-Brain delivery of Temozolamide (TMZ) in brain tumor therapy.Int J Pharm. 2025 Apr 30;675:125540. doi: 10.1016/j.ijpharm.2025.125540. Epub 2025 Mar 31. Int J Pharm. 2025. PMID: 40174811 Review.
-
Improving temozolomide biopharmaceutical properties in glioblastoma multiforme (GBM) treatment using GBM-targeting nanocarriers.Eur J Pharm Biopharm. 2021 Nov;168:76-89. doi: 10.1016/j.ejpb.2021.08.011. Epub 2021 Aug 28. Eur J Pharm Biopharm. 2021. PMID: 34461214 Review.
Cited by
-
Advances in brain-targeted delivery strategies and natural product-mediated enhancement of blood-brain barrier permeability.J Nanobiotechnology. 2025 May 26;23(1):382. doi: 10.1186/s12951-025-03415-w. J Nanobiotechnology. 2025. PMID: 40420216 Free PMC article. Review.
-
Reactive Oxygen Species-Responsive Ferrocene Nanoparticles Delivering Small Interfering RNA Targeting NOP2/Sun RNA Methyltransferase Family Member 2 for Gastric Cancer Therapy.Biomater Res. 2025 May 29;29:0209. doi: 10.34133/bmr.0209. eCollection 2025. Biomater Res. 2025. PMID: 40444266 Free PMC article.
References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
Grants and funding
- No. 82060719/National Natural Science Foundation of China
- NO. CXTD22006/Chinese Medicine Preparation Technology and Equipment Innovation Team of the Jiangxi Uni-versity of Chinese Medicine
- NO. JZYC23S67 and NO. 202410412255/Innovation Project for College Students in Jiangxi University of Chinese Medicine
LinkOut - more resources
Full Text Sources

