Optimization of Albuterol Delivery via Anesthesia Bag in Pediatric Critical Care
- PMID: 40006585
- PMCID: PMC11858898
- DOI: 10.3390/pharmaceutics17020218
Optimization of Albuterol Delivery via Anesthesia Bag in Pediatric Critical Care
Abstract
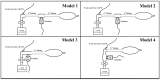
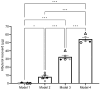
Background/Objectives: Aerosolized medications are common practice for mechanically ventilated pediatric patients. Infants often receive nebulized medications via hand ventilation using an anesthesia bag, but evidence on optimal aerosol delivery with this method is limited. For this study, various configurations of the Mapleson breathing circuit were tested to optimize albuterol delivery to a simulated pediatric model. Methods: Using a simulated pediatric lung model (ASL 5000) with the semi-open Mapleson anesthesia circuit, 2.5 mg/3 mL of albuterol sulfate solution was nebulized to a viral/bacterial filter (Respiguard 202). Four models were compared with varying fresh gas flows (FGFs), small-volume nebulizer (SVN) placements, and adjusting dead space. Five Registered Respiratory Therapists (RRTs) bagged the aerosol into a collection filter following defined ventilation parameters. Each model was tested in random order to avoid fatigue bias. Albuterol concentrations eluted from in-line filters were measured by spectrophotometry (absorbance at 276 nm). Results: No inter-user variability was observed among the RRTs. Significant differences in albuterol recovered were noted between models (One Way ANOVA, Tukey's post hoc, n = 5). Model 4, with the nebulizer closest to the collecting filter, recovered 21.77 ± 1.89% of albuterol. The standard clinical model was the least effective, with only 0.10 ± 0.17% albuterol recovery. Conclusions: Modifying the anesthesia breathing circuit significantly improved aerosol drug delivery efficiency. Our findings suggest that current clinical practices for nebulized drug delivery are inefficient and can be markedly improved with simple adjustments in nebulizer positioning and gas flow within the circuit.
Keywords: manual ventilation; mechanical ventilation; nebulization; pediatric aerosol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A survey of albuterol administration practices in intubated patients in the neonatal intensive care unit.Respir Care. 2002 Jan;47(1):31-8. Respir Care. 2002. PMID: 11749685
-
Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation.Respir Care. 2010 Jul;55(7):845-51. Respir Care. 2010. PMID: 20587095
-
Albuterol Delivery Efficiency in a Pediatric Model of Noninvasive Ventilation With Double-Limb Circuit.Respir Care. 2018 Feb;63(2):141-146. doi: 10.4187/respcare.05833. Epub 2017 Nov 7. Respir Care. 2018. PMID: 29114011
-
Aerosol therapy through high flow nasal cannula in pediatric patients.Expert Rev Respir Med. 2017 Dec;11(12):945-953. doi: 10.1080/17476348.2017.1391095. Epub 2017 Oct 16. Expert Rev Respir Med. 2017. PMID: 28994337 Review.
-
Not all nebulizers are created equal: Considerations in choosing a nebulizer for aerosol delivery during mechanical ventilation.Expert Rev Respir Med. 2023 Feb;17(2):131-142. doi: 10.1080/17476348.2023.2183194. Epub 2023 Feb 27. Expert Rev Respir Med. 2023. PMID: 36803134 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

