Development of a Recombinase-Mediated Cassette Exchange System for Gene Knockout and Expression of Non-Native Gene Sequences in Rickettsia
- PMID: 40006656
- PMCID: PMC11861799
- DOI: 10.3390/vaccines13020109
Development of a Recombinase-Mediated Cassette Exchange System for Gene Knockout and Expression of Non-Native Gene Sequences in Rickettsia
Abstract
Background/objectives: Incidence of vector-borne diseases, including rickettsioses and anaplasmosis, has been increasing in many parts of the world. The obligate intracellular nature of rickettsial pathogens has hindered the development of robust genetic tools for the study of gene function and the identification of therapeutic targets. Transposon mutagenesis has contributed to recent progress in the identification of virulence factors in this important group of pathogens.
Methods: Combining the efficiency of the himar1 transposon method with a recombinase-mediated system, we aimed to develop a genetic tool enabling the exchange of the transposon with a cassette encoding non-native sequences.
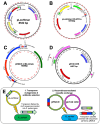
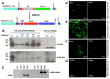
Results: This approach was used in Rickettsia parkeri to insert a himar1 transposon encoding fluorescent protein and antibiotic resistance genes for visualization and selection, flanked by mismatched loxP sites to enable subsequent recombinase-mediated cassette exchange (RMCE). RMCE mediated by a plasmid-encoded Cre recombinase was then employed to replace the transposon with a different cassette containing alternate fluorescent and selection markers and epitopes of Anaplasma phagocytophilum antigens. The resulting genetically modified R. parkeri was trialed as a live-attenuated vaccine against spotted fever rickettsiosis and anaplasmosis in mice.
Conclusions: The use of this system provides a well-established and relatively efficient way of inserting non-native sequences into the rickettsial genome, with applications for the study of gene function and vaccine development.
Keywords: Anaplasma; Rickettsia; genetic tools; live-attenuated vaccine; transposon mutagenesis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Rickettsia parkeri with a Genetically Disrupted Phage Integrase Gene Exhibits Attenuated Virulence and Induces Protective Immunity against Fatal Rickettsioses in Mice.Pathogens. 2021 Jun 30;10(7):819. doi: 10.3390/pathogens10070819. Pathogens. 2021. PMID: 34208806 Free PMC article.
-
Asymptomatic-anaplasmosis confirmation using genetic and serological tests and possible coinfection with spotted fever group Rickettsia: a case report.BMC Infect Dis. 2020 Jun 30;20(1):458. doi: 10.1186/s12879-020-05170-9. BMC Infect Dis. 2020. PMID: 32605544 Free PMC article.
-
Cre/lox-Recombinase-Mediated Cassette Exchange for Reversible Site-Specific Genomic Targeting of the Disease Vector, Aedes aegypti.Sci Rep. 2017 Mar 7;7:43883. doi: 10.1038/srep43883. Sci Rep. 2017. PMID: 28266580 Free PMC article.
-
Recombinase-mediated cassette exchange (RMCE): traditional concepts and current challenges.J Mol Biol. 2011 Mar 25;407(2):193-221. doi: 10.1016/j.jmb.2011.01.004. Epub 2011 Jan 15. J Mol Biol. 2011. PMID: 21241707 Review.
-
The inoculation eschar of Rickettsia parkeri rickettsiosis in Brazil: Importance and cautions.Ticks Tick Borne Dis. 2023 Mar;14(2):102127. doi: 10.1016/j.ttbdis.2023.102127. Epub 2023 Jan 20. Ticks Tick Borne Dis. 2023. PMID: 36693294 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

