A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7
- PMID: 40006671
- PMCID: PMC11860786
- DOI: 10.3390/vaccines13020124
A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7
Abstract
Background/objectives: Enterohemorrhagic Escherichia coli (EHEC) O157:H7, a zoonotic pathogen primarily found in cattle, causes Hemolytic Uremic Syndrome (HUS) in humans, often through contaminated food. Its Type Three Secretion System (T3SS) facilitates gut colonization. In contrast, neonatal calf diarrhea (NCD) is mainly caused by pathogens like enterotoxigenic Escherichia coli (ETEC), Salmonella spp., Bovine Coronavirus (BCoV), and Bovine Rotavirus type A (BRoVA). This study engineered a chimeric protein combining EspB and Int280γ, two T3SS components, expressed in the membranes of Salmonella Dublin and ETEC.
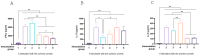
Methods: Immune responses in vaccinated mice and guinea pigs were assessed through ELISA assays.
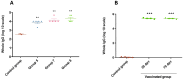
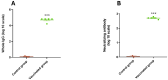
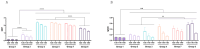
Results: Successful membrane anchorage and stability of the chimera were confirmed. Immune evaluations showed no enhancement from combining recombinant bacteria, indicating either bacterium suffices in a single formulation. Chimeric expression yielded immunogenicity equivalent to 10 µg of recombinant protein, with similar antibody titers. IgG1/IgG2a levels and Th1, Th2, and Th17 markers indicated a mixed immune response, providing broad humoral and cellular protection. Responses to BCoV, BRoVA, ETEC, and Salmonella antigens remained strong and did not interfere with chimera-specific responses, potentially boosting NCD vaccine efficacy.
Conclusions: The chimera demonstrated robust immunogenicity, supporting its potential as a viable vaccine candidate against EHEC O157:H7. This approach could enhance NCD vaccine valency by offering broader protection against calf diarrhea while reducing HUS transmission risks to humans.
Keywords: EHEC O157:H7; ETEC; EspB; Salmonella; bovine vaccine; coronavirus; immune response; intimin; rotavirus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Naylor S.W., Low J.C., Besser T.E., Mahajan A., Gunn G.J., Pearce M.C., Mckendrick I.J., Smith D.G.E., Gally D.L. Lymphoid Follicle-Dense Mucosa at the Terminal Rectum Is the Principal Site of Colonization of Enterohemorrhagic Escherichia coli O157:H7 in the Bovine Host. Infect. Immun. 2003;71:1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

