Cross-Reactive Fc-Mediated Antibody Responses to Influenza HA Stem Region in Human Sera Following Seasonal Vaccination
- PMID: 40006687
- PMCID: PMC11860798
- DOI: 10.3390/vaccines13020140
Cross-Reactive Fc-Mediated Antibody Responses to Influenza HA Stem Region in Human Sera Following Seasonal Vaccination
Abstract
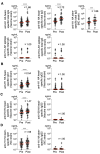
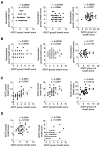
Background: Current influenza A vaccines primarily induce neutralizing antibodies targeting the variable hemagglutinin (HA) head domain, limiting their effectiveness against diverse or emerging influenza A virus (IAV) subtypes. The conserved HA stem domain, particularly the long α-helix (LAH) epitope, is a focus of universal vaccine research due to its cross-protective potential. Additionally, Fc-mediated functions such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) are recognized as important protective immune mechanisms. This study evaluated IgG responses to the HA head, stem, and LAH regions and assessed cross-reactive potential through neutralization, ADCC, and ADCP assays. Methods: IgG responses to the HA head, stem, and LAH regions were measured in vaccinated individuals. Functional assays were conducted for neutralization, ADCC, and ADCP to evaluate the association between antibody levels and immune function. Results: The results showed that HA head-specific IgG increased significantly after vaccination in 50 individuals, whereas stem-specific IgG increased by 72% and LAH-specific IgG by 12-14%. Among the induced antibody subclasses, IgG1 was predominantly increased. Neutralization titers were detected in viruses of the same strain as the vaccine strain, but not in classical or pandemic strains (H5N1, H7N9). HA stem-specific IgG1 antibody titers showed a significant correlation with ADCC/ADCP activity breadth, but no correlation was observed with neutralization breadth. Conclusions: These findings suggest that although current influenza vaccines can induce HA stem-targeted cross-reactive antibodies, their quantity may be insufficient for broad cross-protection, underscoring the need for improved vaccine strategies.
Keywords: antibody-dependent cellular cytotoxicity; antibody-dependent cellular phagocytosis; influenza virus; vaccine.
Conflict of interest statement
Ayae Nishiyama, Takuto Nogimori, Yuji Masuta, Tomoka Matsuura, Tetsuo Kase, Kyoko Kondo, Yu Nakagama, Natsuko Kaku, Sachie Nakagama, Yuko Nitahara, Yoshimasa Takahashi, Hiroshi Kakeya, Yasutoshi Kido, Wakaba Fukushima, and Takuya Yamamoto declare no conflicts of interest. Satoko Ohfuji has not received funding for this study but has received other contract research funding (a retrospective survey on the prevention of herpes zoster through the VARICELLA VACCINE LIVE ATTENUATED “BIKEN”) from The Research Foundation for Microbial Diseases of Osaka University, the manufacturer and distributor of the influenza vaccine.
Figures



Similar articles
-
Measuring influenza hemagglutinin (HA) stem-specific antibody-dependent cellular cytotoxicity (ADCC) in human sera using novel stabilized stem nanoparticle probes.Vaccine. 2020 Jan 22;38(4):815-821. doi: 10.1016/j.vaccine.2019.10.093. Epub 2019 Nov 15. Vaccine. 2020. PMID: 31735504
-
Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques.J Virol. 2014 Nov;88(22):13418-28. doi: 10.1128/JVI.01219-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210172 Free PMC article.
-
Influenza Vaccination Results in Differential Hemagglutinin Stalk-Specific Fc-Mediated Functions in Individuals Living With or Without HIV.Front Immunol. 2022 Apr 19;13:873191. doi: 10.3389/fimmu.2022.873191. eCollection 2022. Front Immunol. 2022. PMID: 35514992 Free PMC article.
-
ADCC: An underappreciated correlate of cross-protection against influenza?Front Immunol. 2023 Feb 22;14:1130725. doi: 10.3389/fimmu.2023.1130725. eCollection 2023. Front Immunol. 2023. PMID: 36911705 Free PMC article. Review.
-
Fc or not Fc; that is the question: Antibody Fc-receptor interactions are key to universal influenza vaccine design.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-9. doi: 10.1080/21645515.2017.1290018. Epub 2017 Mar 23. Hum Vaccin Immunother. 2017. PMID: 28332900 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

