Safety and Immunogenicity of Concomitant Administration and Combined Administration of Bivalent BNT162b2 COVID-19 Vaccine and Bivalent RSVpreF Respiratory Syncytial Virus Vaccine with or Without Quadrivalent Influenza Vaccine in Adults ≥ 65 Years of Age
- PMID: 40006705
- PMCID: PMC11860858
- DOI: 10.3390/vaccines13020158
Safety and Immunogenicity of Concomitant Administration and Combined Administration of Bivalent BNT162b2 COVID-19 Vaccine and Bivalent RSVpreF Respiratory Syncytial Virus Vaccine with or Without Quadrivalent Influenza Vaccine in Adults ≥ 65 Years of Age
Abstract
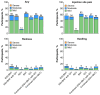
Concomitant administration may improve vaccination rates. This analysis of a phase 1/2 randomized study included 1073 healthy ≥65-year-olds who previously received ≥3 mRNA COVID-19 vaccine doses. Participants received concomitantly administered RSVpreF and bivalent BA.4/BA.5-adapted BNT162b2 vaccine (concomitant administration) with or without quadrivalent influenza vaccine (QIV), admixed combined RSVpreF + BNT162b2 vaccine (combined vaccine) with or without QIV, RSVpreF, BNT162b2, or QIV. Immunogenicity objectives included demonstrating the noninferiority of neutralizing antibody titers elicited by concomitant administration and combined vaccine compared with RSVpreF or BNT162b2 administered alone, and by concomitant administration and combined vaccine given with QIV compared with RSVpreF, BNT162b2, and QIV alone. Reactogenicity (≤7 days) and safety ≤1 month (adverse events (AEs)) and ≤6 months (serious AEs (SAEs)) after vaccination were assessed. Noninferiority for all immunogenicity comparisons was demonstrated. All vaccine groups were well tolerated; no new safety concerns were identified. Reactogenicity was mostly mild/moderate with rates generally similar across groups, except injection site pain and fatigue, which were less frequent with RSVpreF + placebo vs. other groups. AEs were infrequent, mostly mild/moderate, occurring at similar frequencies across groups. No AEs leading to study withdrawal or vaccine-related SAEs were reported. Favorable safety and tolerability alongside similar immunogenicity provide support for concomitant or combined use of RSVpreF and BNT162b2, with or without QIV, to help protect older adults from these important respiratory pathogens (NCT05886777).
Keywords: BNT162b2; QIV; RSVpreF; concomitant; immunogenicity; noninferiority; safety.
Conflict of interest statement
Joel M. Neutel, Kevin Cannon, and Helen Stacey have no conflicts to declare. Federico J. Mensa, Özlem Türeci, and Uğur Şahin are BioNTech employees and own stock or stock options. All other authors are employees of Pfizer and may own stock or stock options.
Figures





References
-
- Chadha M., Hirve S., Bancej C., Barr I., Baumeister E., Caetano B., Chittaganpitch M., Darmaa B., Ellis J., Fasce R., et al. Human respiratory syncytial virus and influenza seasonality patterns-Early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir. Viruses. 2020;14:638–646. doi: 10.1111/irv.12726. - DOI - PMC - PubMed
-
- World Health Organization . Respiratory Syncytial Virus (RSV) Disease. World Health Organization; Geneva, Switzerland: 2023.
-
- Troeger C.E., Blacker B.F., Khalil I.A., Zimsen S.R., Albertson S.B., Abate D., Abdela J., Adhikari T.B., Aghayan S.A., Agrawal S., et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. - DOI - PMC - PubMed
-
- US Centers for Disease Control and Protection Surveillance of RSV. [(accessed on 29 January 2025)]; Available online: https://www.cdc.gov/rsv/php/surveillance/index.html#:~:text=Surveillance...
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

