SARS-CoV-2 Impairs Osteoblast Differentiation Through Spike Glycoprotein and Cytokine Dysregulation
- PMID: 40006897
- PMCID: PMC11860324
- DOI: 10.3390/v17020143
SARS-CoV-2 Impairs Osteoblast Differentiation Through Spike Glycoprotein and Cytokine Dysregulation
Abstract
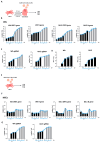
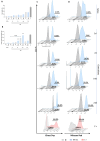
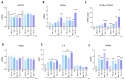
Pulmonary and extrapulmonary manifestations have been reported following infection with SARS-CoV-2, the causative agent of COVID-19. The virus persists in multiple organs due to its tropism for various tissues, including the skeletal system. This study investigates the effects of SARS-CoV-2 infection, including both ancestral and Omicron viral strains, on differentiating mesenchymal stem cells (MSCs), the precursor cells, into osteoblasts. Although both viral strains can productively infect osteoblasts, precursor cell infection remained abortive. Viral exposure during osteoblast differentiation demonstrates that both variants inhibit mineral and organic matrix deposition. This is accompanied by reduced expression of runt-related transcription factor 2 (RUNX2) and increased levels of interleukin-6 (IL-6), a cytokine that negatively regulates osteoblast differentiation. Furthermore, the upregulation of receptor activator of nuclear factor kappa B ligand (RANKL) strongly suggests that the ancestral and Omicron variants may disrupt bone homeostasis by promoting osteoclast differentiation, ultimately leading to the formation of bone-resorbing cells. This process is dependent of spike glycoprotein since its neutralization significantly reduced the effect of infective SARS-CoV-2 and UV-C inactivated virus. This study underscores the capacity of ancestral and Omicron SARS-CoV-2 variants to disrupt osteoblast differentiation, a process essential for preserving the homeostasis and functionality of bone tissue.
Keywords: COVID-19; SARS-CoV-2; bone; osteoblasts.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
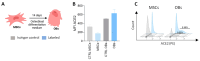

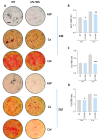
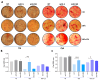
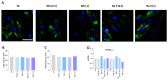
References
-
- Buckwalter J.A., Cooper R.R. Bone structure and function. Instr. Course Lect. 1987;36:27–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

